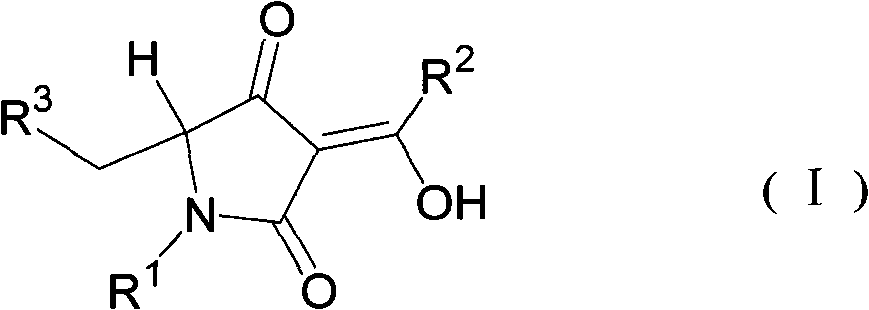
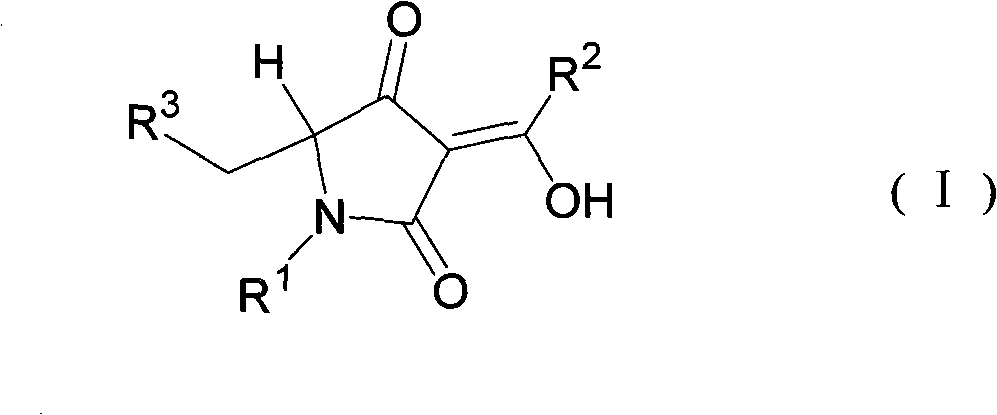
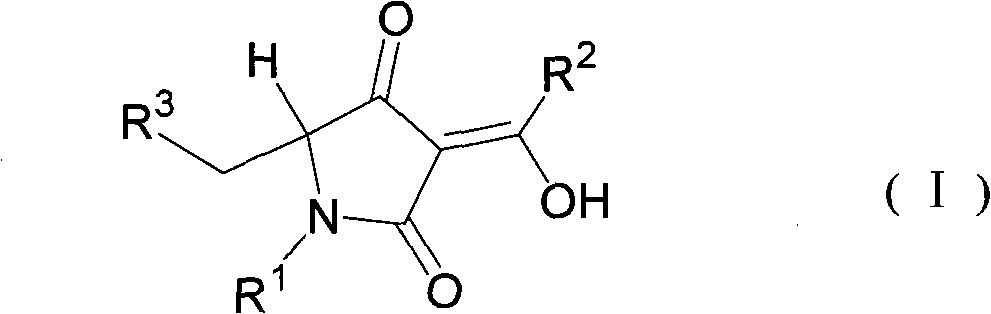
5-substituted methylpyrrolidine-2, 4-diketone compound and use thereof
A kind of technology of methyl pyrrolidine and compound, applied in the field of herbicide preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of 3-(1-hydroxyethylidene)-5-(4-methoxybenzoylmethyl)pyrrolidine-2,4-dione (6f)
[0042] Equipped with thermometer, electric stirring (sealed), constant pressure dropping funnel, with CaCl 2 Add methyl N-acetoacetyl-2-(4-methoxybenzoylmethyl)-2-aminoacetate (0.1mol), 30mL methanol, sodium methoxide ( 2.3gNa+30mL methanol), refluxed for 2h, desolvated under reduced pressure, added 50mL of water, and repeatedly extracted with ethyl acetate to remove impurities until the extract was basically colorless. Acidify to pH 2 with 3M HCl, extract with ethyl acetate, anhydrous MgSO 4 Drying, suction filtration, precipitation, the residue was recrystallized from methanol to give a yellow solid 3-(1-hydroxyethylidene)-5-(4-methoxybenzoylmethyl)pyrrolidine-2,4-di Ketones (6f). Its physical parameters and spectral data are:
[0043] 3-(1-Hydroxyethylidene)-5-(4-methoxybenzoylmethyl)pyrrolidine-2,4-dione (6f): Yield: 71%; Yollow powder; m.p.160.6-162.0 °C; I...
Embodiment 2
[0047] Example 2: Preparation of 2-(4-(1-hydroxyethylidene)-3,5-dioxypyrrolidin-2-yl)butyl acetate (12a)
[0048] Equipped with thermometer, electric stirring (sealed), water separator, with CaCl 2 Add 2-(4-(1-hydroxyethylidene)-3,5-dioxypyrrolidin-2-yl)acetic acid (0.1mol), several drops of concentrated sulfuric acid, Butanol (0.4mol), 30mL benzene and 10mL DMF, stirred, heated, refluxed for 4h, desolventized, and the residue was recrystallized with methanol to obtain a white solid 2-(4-(1-hydroxyethylidene)-3, 5-Dioxypyrrolidin-2-yl)butyl acetate (12a). Its physical parameters and spectral data are:
[0049] 2-(4-(1-hydroxyethylidene)-3,5-dioxypyrrolidin-2-yl)butyl acetate (12a).Yeild: 80%; Colorless powder; m.p.61.8-62.3℃; (c 0.50, methanol); IR (KBr, cm -1 )v: 3428, 3216, 3060, 2958, 2935, 2873, 1738, 1710, 1664, 1620, 1451, 1331, 1219, 1175, 899, 733, 645; 1 H NMR (400MHz, CDCl 3 )δ (ppm): 6.37 (br, 1H, CHNH), 4.16-4.14 (m, 3H, CHNH+OCH 2 ), 3.03 (d, J=17.3Hz, 1H,...
Embodiment 3
[0060] Example 3: Preparation of 3-(1-hydroxyethylidene)-5-(butylthiomethyl)pyrrolidine-2,4-dione (18a)
[0061] Equipped with thermometer, electric stirring (sealed), constant pressure dropping funnel, with CaCl 2 Add N-acetoacetyl-2-butylthiomethyl-2-aminoacetic acid methyl ester (0.1mol), 30mL methanol, sodium methoxide (2.3g Na+30mL methanol) into the 250mL four-necked flask of the reflux tube of the drying tube , refluxed for 2 hours, desolvated under reduced pressure, added 50 mL of water, and extracted repeatedly with ethyl acetate to remove impurities until the extract was basically colorless. Acidify to pH 2 with 3M HCl, extract with ethyl acetate, Na 2 SO 4 After drying, suction filtration and solvent extraction, the residue was recrystallized from methanol to obtain 3-(1-hydroxyethylidene)-5-(butylthiomethyl)pyrrolidine-2,4-dione (18a) as a white solid. Its physical parameters and spectral data are:
[0062] 3-(1-Hydroxyethylidene)-5-(butylthiomethyl)pyrrolidine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com