Enhancement of drug therapy by mirna
A therapeutic and biological technology that can be applied to DNA/RNA fragments, drug combinations, recombinant DNA technology, etc., and can solve problems such as interference with mitosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
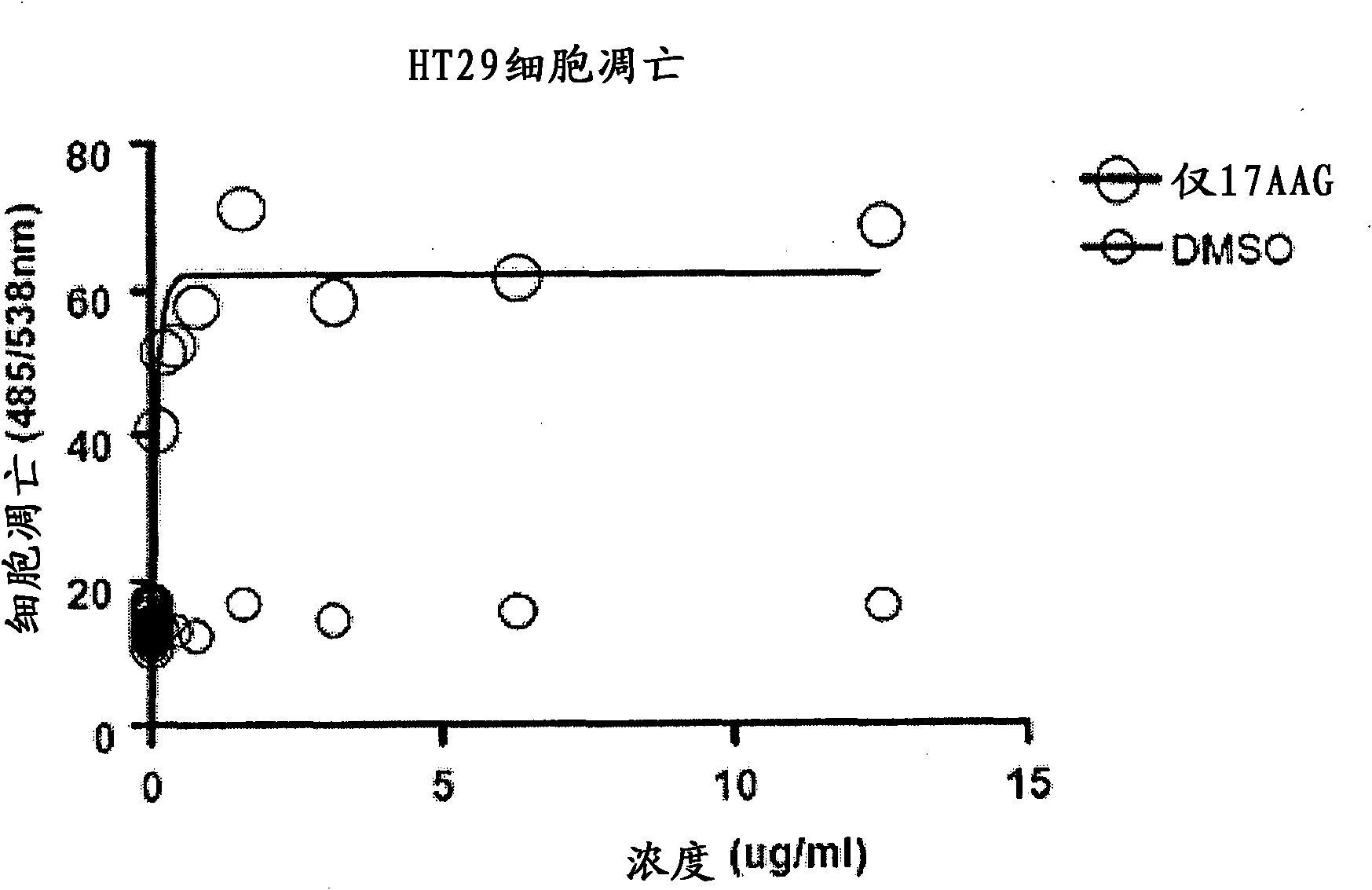
[0215] This example demonstrates the apoptotic activity of the HSP90 inhibitor 17-AAG.
[0216] The homogeneous caspase-3 / 7 assay (Promega, Madison, WI) uses a proprietary lysis / activation buffer together with (Z-DEVD) 2-rhodamine 110 substrate, allowing for use in adherent, A simple "add-mix-read" format for the detection of caspase-3 and -7 in suspension and primary culture cells or in purified caspase preparations. The assay uses a rhodamine 110-based substrate that allows for extreme sensitivity previously unobtainable with conventional colorimetric or fluorometric assays.
[0217] In particular, 100 μl Caspase-3 / 7 reagent was added to each well of a white or black 96-well plate containing 100 μl of blank, control or cultured cells. Cover the plate with a plate sealer for extended periods of incubation (>4 hours). To perform the assay in a 384-well plate, the Caspase-3 / 7 reagent:sample 1:1 volume ratio. The contents of the wells were mixed using a plate shaker at ...
Embodiment 2
[0220] This example demonstrates that the HSP90 inhibitor 17-AAG inhibits Her2 expression.
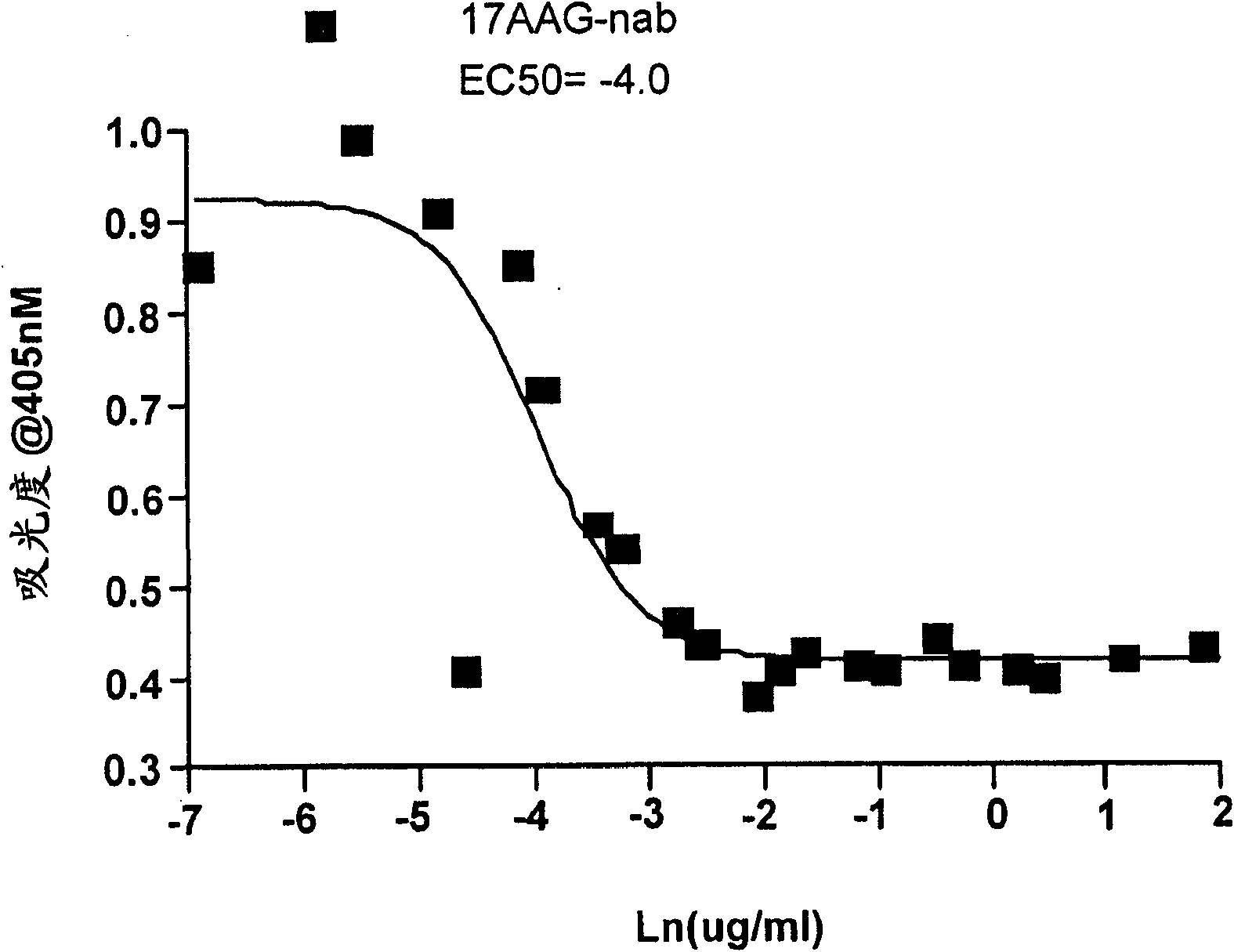
[0221] 10,000 BT474 cells / well were seeded into microtiter plates and grown for 48 hours. After this pre-incubation, BT474 cells were treated with various concentrations of 17-AAG and its analogs for 24 hours. At the end of this incubation, medium was removed from each well, each well was washed twice with ice-cold Tris-buffered saline (containing 0.1% Tween 20), and cells were fixed with methanol (ice-cold) at 40°C 10 minutes. Fixed BT474 cells were immunostained with anti-Her2 antibody. The presence of Her2 protein was determined by measuring the absorbance at 405 nm in a plate reader.
[0222] like figure 2 As shown in , the IC50 for 17-AAG is close to 32 nM for the Her2 inhibition assay. This result suggests that 17-AAG strongly inhibits Her2 protein expression.
Embodiment 3
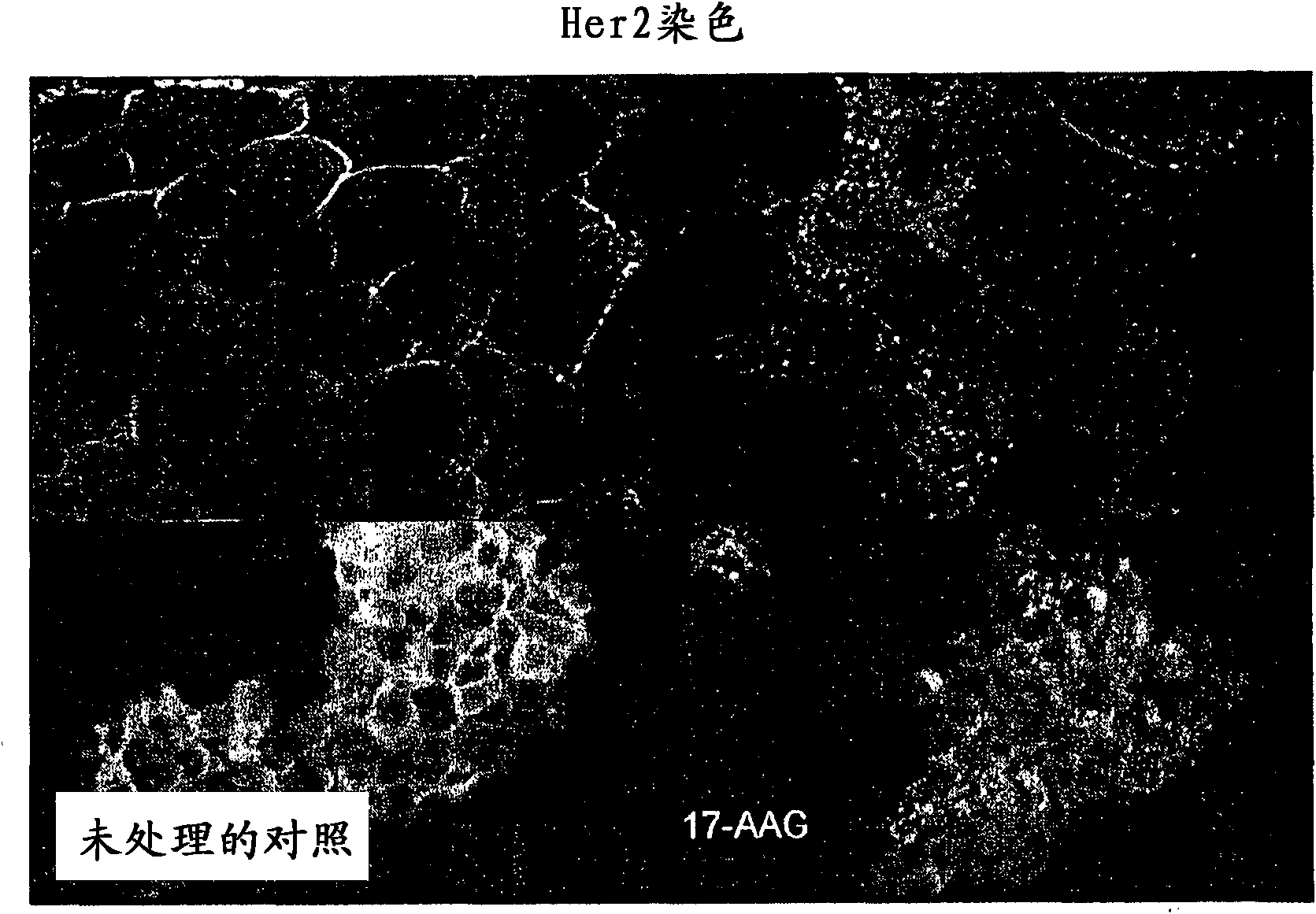
[0224] This example demonstrates the internalization and degradation of Her2 following 17-AAG inhibition of HSP90.
[0225] 17-AAG-treated BT474 cells were examined by confocal imaging system. BT474 cells were seeded on glass slides and treated at IC50 concentrations for 24 hours. 17-AAG-treated and control BT474 cells were fixed with methanol, stained with Her2 antibody, and analyzed by confocal imaging. like image 3 As shown in , after 17-AAG treatment, Her2 protein expression was abolished from its cell surface sublocalization and sublocalized to the cytoplasm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com