Tetrafluorophenoxy nicotinamide compound as well as preparation method and application thereof for sterilizing
A technology of tetrafluorophenoxynicotine amine and compounds, which is applied in the field of crop bactericidal compounds, can solve problems such as unsatisfactory control effects, and achieve good bactericidal effects, long-lasting effects, and high therapeutic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
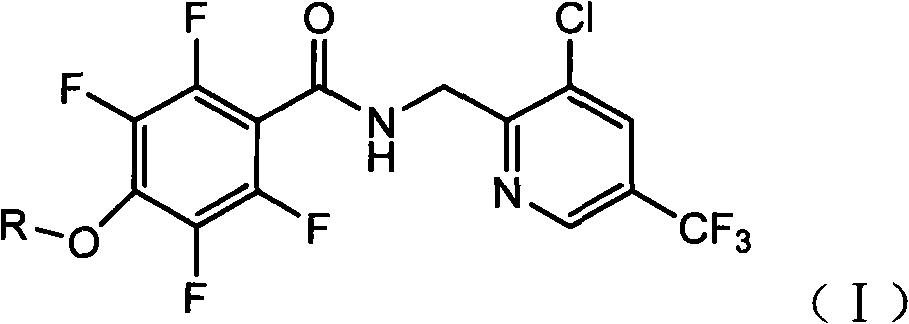
[0016] Compound 1: N-(3-chloro-5-trifluoromethyl)pyridin-2-yl)methyl)-2,3,5,6-tetrafluoro-4-methoxybenzoic acid carboxamide
[0017] The structural formula is:
[0018]
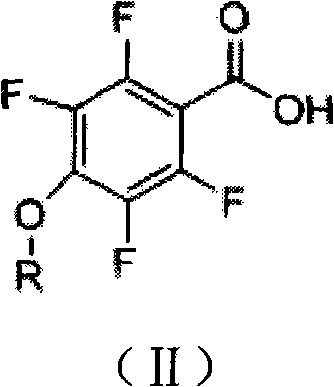
[0019] The preparation method is: put 22.4 grams of 2,3,5,6-tetrafluoro-4-methoxybenzoic acid, 200 ml of dichloromethane, and 15 ml of thionyl chloride into a three-necked flask, and stir at 0°C for 10 hours , the solvent and residual thionyl chloride are distilled off under reduced pressure. During distillation, the vacuum degree is controlled at -0.010kpa, and the temperature is not more than 100 degrees. Amino-3-chloro-5-trifluoromethylpyridine 21.0 g, cool down to 0 °C, add 12.6 ml of triethylamine dropwise at this temperature, after the addition is complete, keep it warm for 20 hours, add 300 ml of water to wash away the reaction formed Triethylamine hydrochloride was washed with 300 ml of water, dried, and the solvent was removed to obtain 38 to 39 grams of off-white solid N-(3-chloro-5-trifluoromet...
specific Embodiment 2
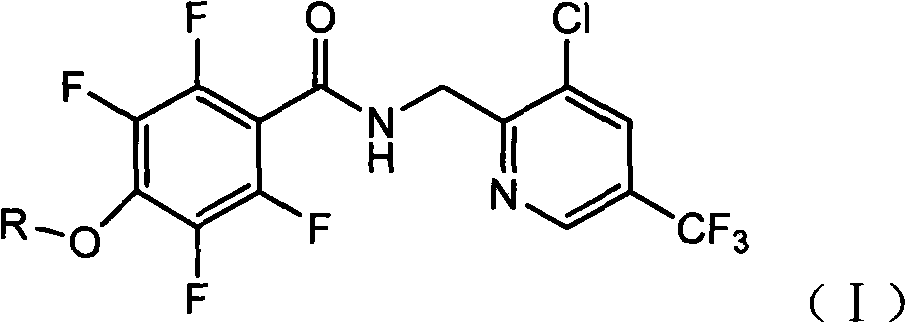
[0021] Compound 2: N-(3-chloro-5-trifluoromethyl)pyridin-2-yl)methyl)-2,3,5,6-tetrafluoro-4-ethoxybenzoic acid carboxamide
[0022] The structural formula is:
[0023]
[0024] The preparation method is: put 23.8 grams of 2,3,5,6-tetrafluoro-4-ethoxybenzoic acid, 200 ml of dichloromethane, and 25 ml of thionyl chloride into a three-necked flask, and stir at 20°C for 24 hours , the solvent and residual thionyl chloride are distilled off under reduced pressure. During distillation, the vacuum degree is controlled at -0.098kpa, and the temperature is not more than 100 degrees. Amino-3-chloro-5-trifluoromethylpyridine 21.0 g, cooled to 5 ° C, at this temperature, 63 ml of triethylamine was added dropwise, after the addition was completed, it was kept for 48 hours, and 300 ml of water was added to wash away the reaction formed Triethylamine hydrochloride, washed with 300 ml of water, dried, and stripped of the solvent gave 40-42 g of N-(3-chloro-5-trifluoromethyl)pyridin-2-yl)m...
specific Embodiment 3
[0026] Compound 3: N-(3-chloro-5-trifluoromethyl)pyridin-2-yl)methyl)-2,3,5,6-tetrafluoro-4-propoxybenzoic acid carboxamide
[0027] The structural formula is:
[0028]
[0029] The preparation method is: put 25.2 grams of 2,3,5,6-tetrafluoro-4-propoxybenzoic acid, 200 ml of dichloromethane, and 20 ml of thionyl chloride into a three-necked flask, and stir at 10°C for 17 hours , the solvent and residual thionyl chloride are distilled off under reduced pressure. During distillation, the vacuum degree is controlled at -0.050kpa, and the temperature is not more than 100 degrees. Amino-3-chloro-5-trifluoromethylpyridine 21.0 g, cool down to 4 °C, add 38 ml of triethylamine dropwise at this temperature, after the addition is complete, keep it warm for 30 hours, add 300 ml of water to wash away the reaction formed Triethylamine hydrochloride, washed with 300 ml of water, dried, and stripped of the solvent gave 42-44 g of N-(3-chloro-5-trifluoromethyl)pyridin-2-yl)methyl )-2,3,5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com