Application of pseudoginseng root total saponins and preparations thereof in adjuvant treatment of 'systemic inflammatory response syndrome'
A technology of Panax notoginseng saponins and adjuvant therapy, applied in the field of prevention or adjuvant therapy of "systemic inflammatory response syndrome," can solve the problems of lack of varieties and large economic burden on patients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
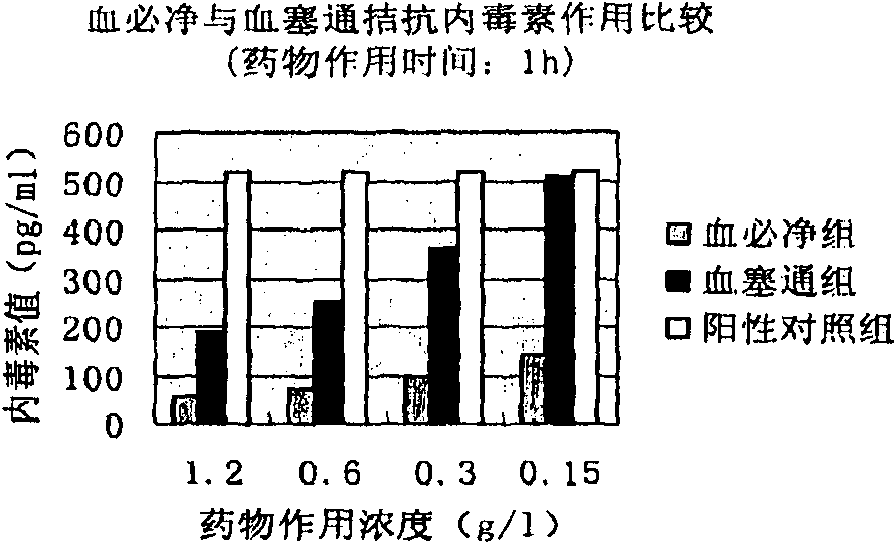
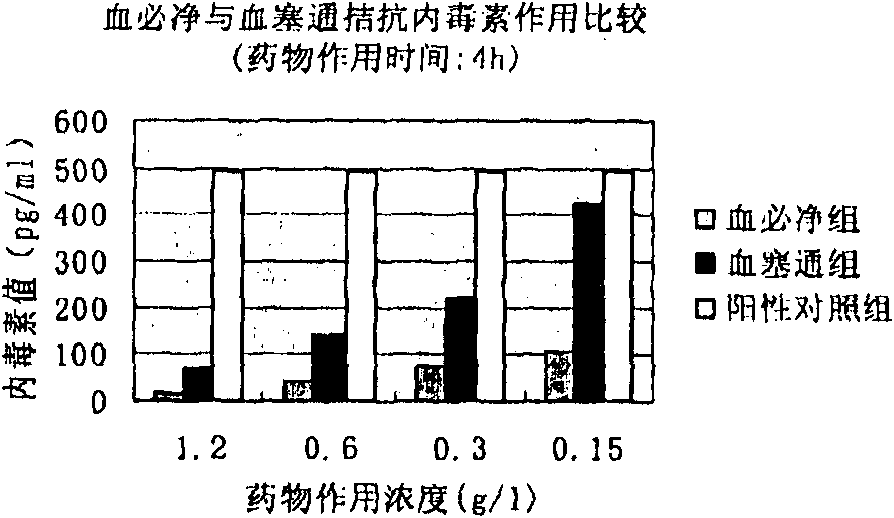
[0050]Example 1 The effect of Xuebijing injection and Xuesaitong powder injection on antagonizing endotoxin in vitro
[0051] Objective: To observe whether the drug has the pharmacodynamic effect of antagonizing endotoxin in vitro by dynamic turbidity method, and to compare the effect with Xuebijing at the same time.
[0052] Dilute Xuebijing (10g / L) and Xuesaitong freeze-dried powder with bacterial endotoxin test water (BET water) to a series of required concentrations: 0.15, 0.3, 0.6 and 1.2g / L; The toxin was dissolved in BET water, vortexed for 15 min and then diluted to a concentration of 5×10 5 EU / L (5×10 per liter 5 endotoxin unit), add 10 μl of diluted endotoxin to 1ml of liquid sample and make the final concentration 5×10 3 Add EU / L toxin and incubate at 37°C for 1 hour or 4 hours. In addition, a positive control group (endotoxin plus BET water) and three negative control groups (no pyrogen water, namely BET water, Xuesaitong freeze-dried powder for injection with a...
Embodiment 2
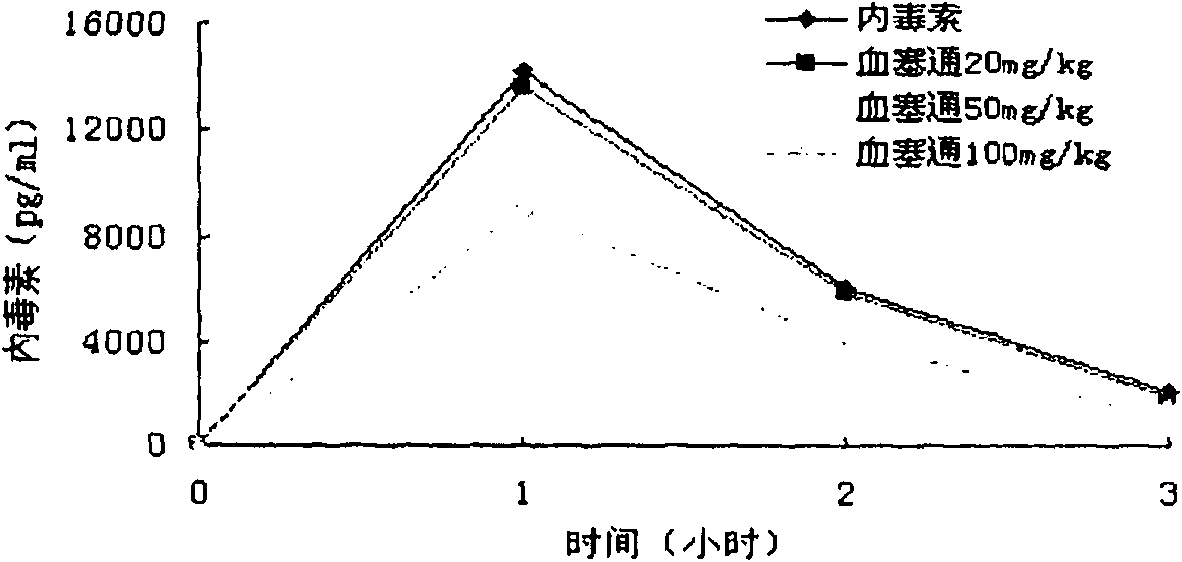
[0059] Example 2 Experimental Study of Xuesaitong Powder Injection Antagonizing Endotoxin in Vivo
[0060] 2.1 Xuesaitong antagonizes the effect of endotoxin (LPS) in rabbits, and compares it with Xuebijing.
[0061] Animals were divided into 6 groups. Five rats in the endotoxin group were given LPS 0.5 mg / kg intravenously. Xuesaitong intervention groups I-III, 5 rats in each group, were given LPS and endotoxin group, and Xuesaitong 20mg / kg, 50mg / kg and 100mg / kg were given after 0.5h. 5 rats in Xuebijing group were given LPS and endotoxin group, and Xuebijing 50mg / kg was given 0.5h later. In the normal control group of 5 rats, LPS 0.5mg / Kg was replaced by normal saline. Each group in the experiment was given blood at 1h, 2h and 4h after administration of LPS or normal saline, and the dynamic turbidity method was used to detect the plasma endotoxin content, and the endotoxin recovery rate was calculated: recovery rate (%)=(endotoxin group value-treatment group value) / endoto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com