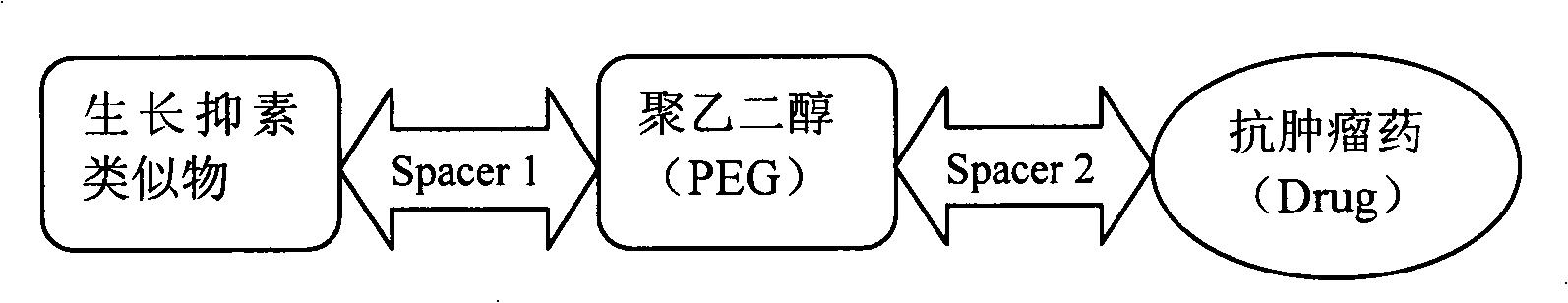
Conjugate of SSA, PEG and anticancer drugs and preparation thereof
A technology of polyethylene glycol and somatostatin, applied in antitumor drugs, drug combinations, pharmaceutical formulations, etc., to achieve the effects of improving targeting, prolonging residence time, and reducing drug toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of octreotide-polyethylene glycol-paclitaxel conjugate (OCT-PEG-PTX) (with succinic anhydride as the linking arm)
[0032] (1) Dissolve 35 mg of paclitaxel (PTX) in pyridine, add 4 mg of succinic anhydride, and stir for 4 hours at room temperature. Pyridine was removed by rotary evaporation and dried in vacuo. Add an appropriate amount of double-distilled water, stir for 30 minutes, collect the precipitate by filtration, dissolve the precipitate in an appropriate amount of acetone, slowly add double-distilled water, collect the crystals, and dry in vacuum to obtain 2'-succinylpaclitaxel. MRI 1 HNMR and mass spectrometry MS determined its structure.
[0033] (2) 2'-succinyl paclitaxel obtained in (1) was dissolved in acetonitrile, and 200 mg H 2 N-PEG-COOH (PEG 5000 ), add 16 mg 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) and 10 mg N-hydroxysuccinimide (NHS), and stir at room temperature for 12 h. Take the above reaction soluti...
Embodiment 2
[0036] Preparation of octreotide-polyethylene glycol-paclitaxel conjugate (OCT-PEG-PTX) (with succinic acid as the linker)
[0037] (1) Dissolve 35 mg of paclitaxel (PTX) in pyridine, add 4 mg of succinic acid, and stir at room temperature for 4 hours. Pyridine was removed by rotary evaporation and dried in vacuo. Add an appropriate amount of double-distilled water, stir for 30 minutes, collect the precipitate by filtration, dissolve the precipitate in an appropriate amount of acetone, slowly add double-distilled water, collect the crystals, and dry in vacuum to obtain 2'-succinylpaclitaxel. MRI 1 HNMR and mass spectrometry MS determined its structure.
[0038] (2) 2'-succinyl paclitaxel obtained in (1) was dissolved in acetonitrile, and 200 mg H 2 N-PEG-COOH (PEG 5000 ), add 16 mg 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) and 10 mg N-hydroxysuccinimide (NHS), and stir at room temperature for 12 h. Take the above reaction solution, add anhydro...
Embodiment 3
[0041] Preparation of vapreotide-polyethylene glycol-paclitaxel conjugate (VAP-PEG-PTX) (with succinic anhydride as the linking arm)
[0042] (1) Dissolve 35 mg of paclitaxel (PTX) in pyridine, add 4 mg of succinic anhydride, and stir for 4 hours at room temperature. Pyridine was removed by rotary evaporation and dried in vacuo. Add an appropriate amount of double-distilled water, stir for 30 minutes, collect the precipitate by filtration, dissolve the precipitate in an appropriate amount of acetone, slowly add double-distilled water, collect the crystals, and dry in vacuum to obtain 2'-succinylpaclitaxel. MRI 1 HNMR and mass spectrometry MS determined its structure.
[0043] (2) 200mg (Boc) HN-PEG-CONHS (PEG 2000 ) was dissolved in an appropriate amount of acetonitrile, 40 mg of vapreotide was dissolved in an appropriate amount of N, N-dimethylformamide (DMF), the PEG solution was added dropwise to the vapreotide solution, and then 5 mg of dimethylaminopyridine (DMAP) was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com