Thermo-sensitive in-situ gel pharmaceutical composition
An in-situ gel and composition technology, which is applied in the field of in-situ gel pharmaceutical compositions, can solve the problems of inability to form temperature-sensitive gels, high cost, and large dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
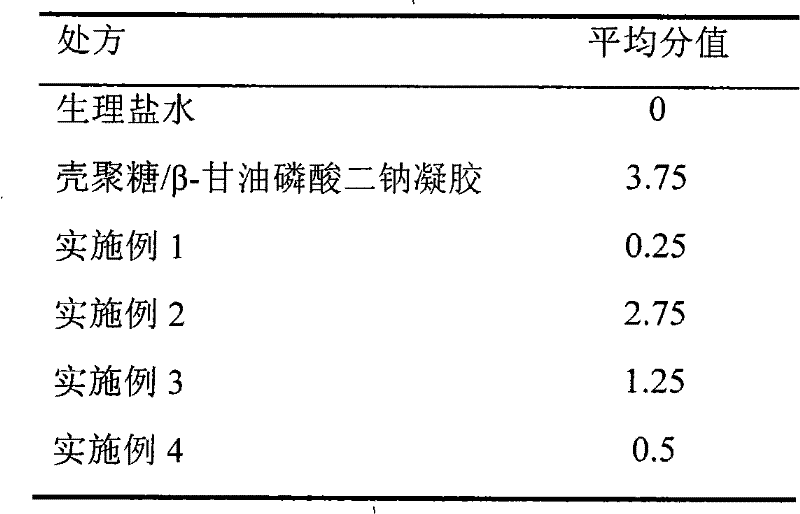
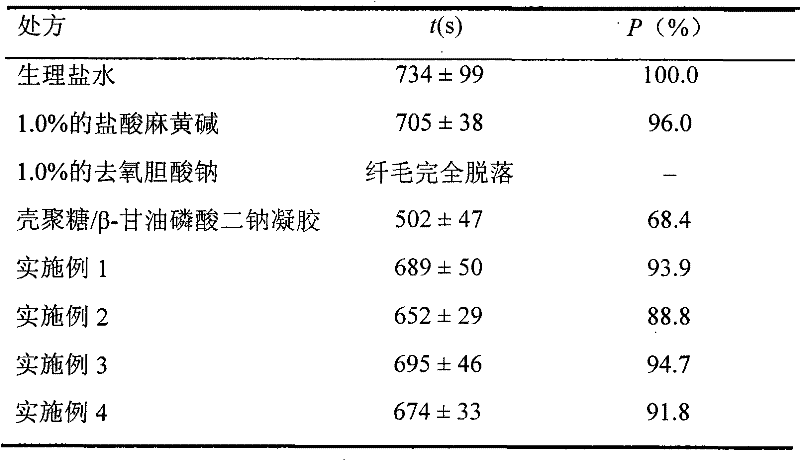
[0020] Embodiment 1: Preparation of temperature-sensitive in-situ gel
[0021] According to 1.32% chitosan (deacetylation degree is 80%, molecular weight 800,000), 18% glucose phosphate, 1% cetirizine hydrochloride weight percentage, chitosan is dissolved in 0.1M hydrochloric acid Neutralize and stir evenly, then add cetirizine hydrochloride, stir to dissolve, add appropriate amount of Tween80, benzalkonium bromide, finally add glucose phosphate, stir evenly, filter, sterilize, can, and seal. Each gram of gel contains 10 mg of cetirizine hydrochloride. The gelation temperature was determined to be 34°C by the test tube inversion method.
Embodiment 2
[0022] Embodiment 2: Preparation of temperature-sensitive in-situ gel
[0023] By 1.32% chitosan (degree of deacetylation is 85%, molecular weight 400,000), 26% sorbitol phosphate, 3% cetirizine hydrochloride by weight percentage, chitosan is dissolved in 0.1M Add in hydrochloric acid and stir evenly, then add cetirizine hydrochloride, stir to dissolve, add appropriate amount of Ploumic F68, thimerosal, and finally add sorbitol phosphate, stir evenly, filter, sterilize, can, and seal. Each gram of gel contains 30 mg of cetirizine hydrochloride. The gelation temperature was determined to be 33°C by the test tube inversion method.
Embodiment 3
[0024] Embodiment 3: Preparation of temperature-sensitive in-situ gel
[0025]According to 1.32% chitosan (deacetylation degree is 82%, molecular weight 550,000), 21.5% fructose phosphate, 2% cetirizine hydrochloride by weight percentage, chitosan is dissolved in 0.1M hydrochloric acid and stir evenly, then add cetirizine hydrochloride, stir to dissolve, add appropriate amount of edetate disodium, ethylparaben, finally add fructose phosphate, stir evenly, filter, sterilize, can, seal Instantly. Each gram of gel contains 20mg of cetirizine hydrochloride. The gelation temperature was determined to be 34°C by the test tube inversion method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com