A54-GFLG-DOX conjugate as well as coupling method and application thereof
A technology of conjugates and liver cancer, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, etc., which can solve the limitations of doxorubicin, doxorubicin myelosuppressive myocardial toxicity and neurotoxicity, and endanger the health of patients and other issues to achieve the effect of effective killing and reducing the killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
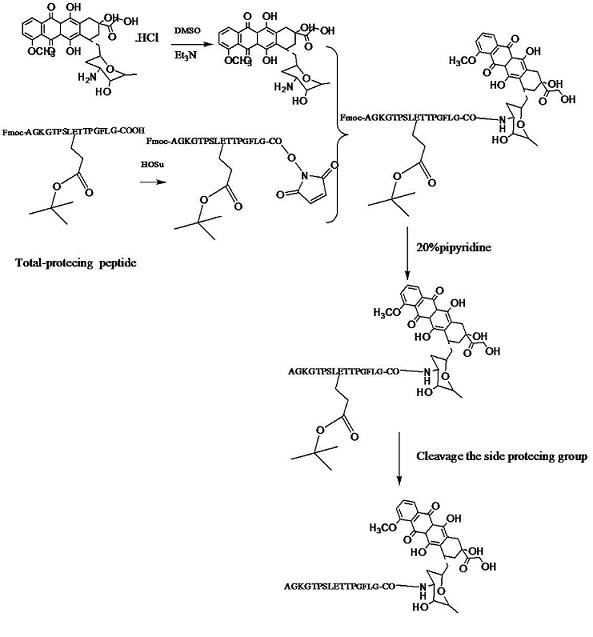
[0056] Example 1: Chemical synthesis of A54-GFLG-DOX conjugate
[0057] The structure of polypeptide A54 (hepatoma specific targeting peptide A54, amino acid sequence: AGKGTPSLETTP) contains two carboxyl groups -COOH, and four amino acid GFLGs are connected to the terminal carboxyl group to obtain A54-GFLG. Using Fmoc to protect the -COOH in the middle of the liver cancer-specific targeting peptide A54, exposing the GFLG terminal -COOH, HoSu activates the polypeptide to neutralize the terminal carboxyl group with triethylamine (Et3N) and doxorubicin hydrochloride- After the NH2 reaction, piperidine removes Fmoc, and the product is purified and separated by preparative HPLC.
[0058] The specific steps are as follows:
[0059] 1. Using conventional peptide synthesis technology, four amino acids G, F, L, and G are sequentially connected to the terminal carboxyl group of polypeptide A54 to form polypeptide A54-GFLG;
[0060] 2. Take 5.8 mg (0.01 mmol) of doxorubicin hydrochloride and di...
Embodiment 2
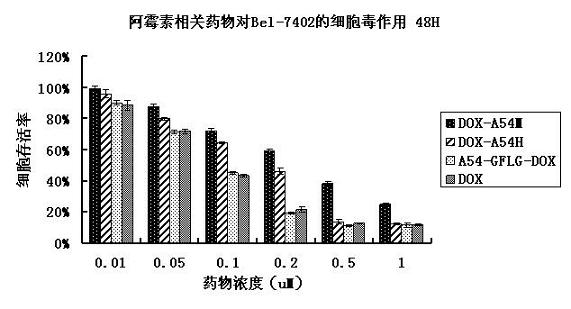
[0065] Example 2: Cytotoxicity detection of A54-GFLG-DOX and DOX-A54H
[0066] In order to detect whether the synthesized A54-GFLG-DOX maintains the killing effect of doxorubicin on liver cancer cells, a cytotoxicity experiment was used to detect the changes in cell viability of the cells after the drug was applied compared to the untreated control group. The specific steps are as follows:
[0067] 1. 0.25% trypsin digests adherent cells BEL-7402, and collects the cells in a serum-containing medium;
[0068] 2. Count the cells, centrifuge, resuspend in the medium, and adjust the cell concentration to 1×10 5 Pcs / ml;
[0069] 3. Inoculate cells in a 96-well cell culture plate and add 90ul of cell suspension to each well. Leave 3 blank holes and add 100ul cell-free medium as a blank control;
[0070] 4. Dilute the drugs A54-GFLG-DOX, DOX-A54H, DOX-A54M and DOX with the culture medium to make a series of 6 low concentrations. Add 10ul of the drug to each hole and 3 replicate holes for eac...
Embodiment 3
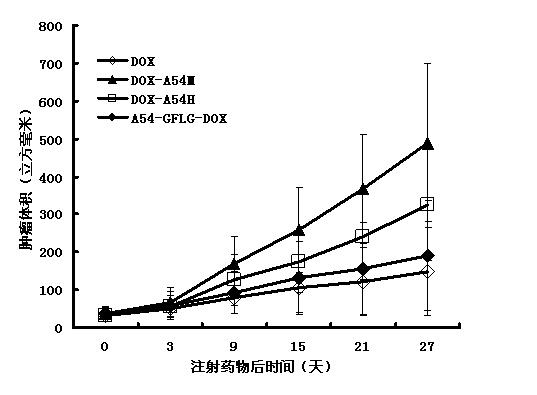
[0076] Example 3: Targeted detection of A54-GFLG-DOX and DOX-A54H
[0077] As a classic antibiotic broad-spectrum anti-tumor drug, doxorubicin molecules inhibit DNA replication by cross-linking with tumor cell DNA, and block the action of RNA polymerase to inhibit RNA synthesis, thereby achieving the purpose of inhibiting tumor growth. And doxorubicin itself has red fluorescence. According to the action mechanism and characteristics of doxorubicin, the present invention adds two synthetic target drugs to liver cancer cell lines. After incubation, observe the red fluorescence of doxorubicin to determine the present invention. The fluorescence intensity of A54-GFLG-DOX conjugate and DOX-A54H is used to determine and compare the activity of the two drugs.
[0078] The specific steps are as follows:
[0079] 1. Seed cells in a 96-well cell culture plate to make the number of cells 1×10 4 Pcs / hole;
[0080] 2. At 37℃, saturated humidity, 5% CO 2 Cultivate in a cell incubator until the cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com