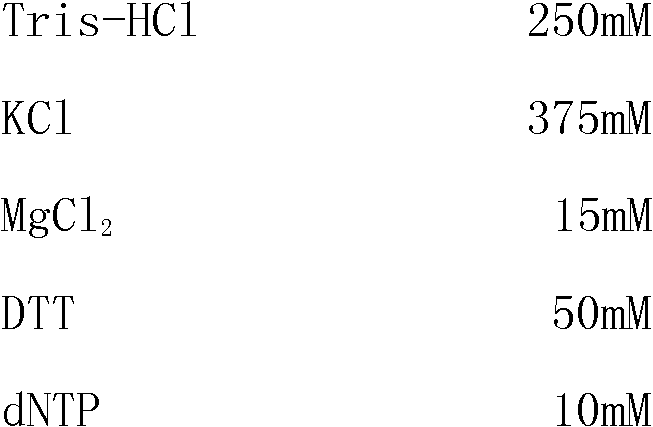RT(Reverse Transcription)-PCR (Polymerase Chain Reaction) kit for detecting parainfluenza virus in one step
An RT-PCR and detection kit technology, applied in the field of one-step RT-PCR detection kits for parainfluenza virus, can solve the problems of human parainfluenza virus infection without vaccine, and achieve short detection time period, high specificity and detection efficiency high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] 1. Example 1---One-step RT-PCR detection kit for parainfection virus
[0068] 12 throat swab samples from patients with suspected parainfection virus infection were detected, and 200 μl of each sample solution was taken for virus nucleic acid extraction.
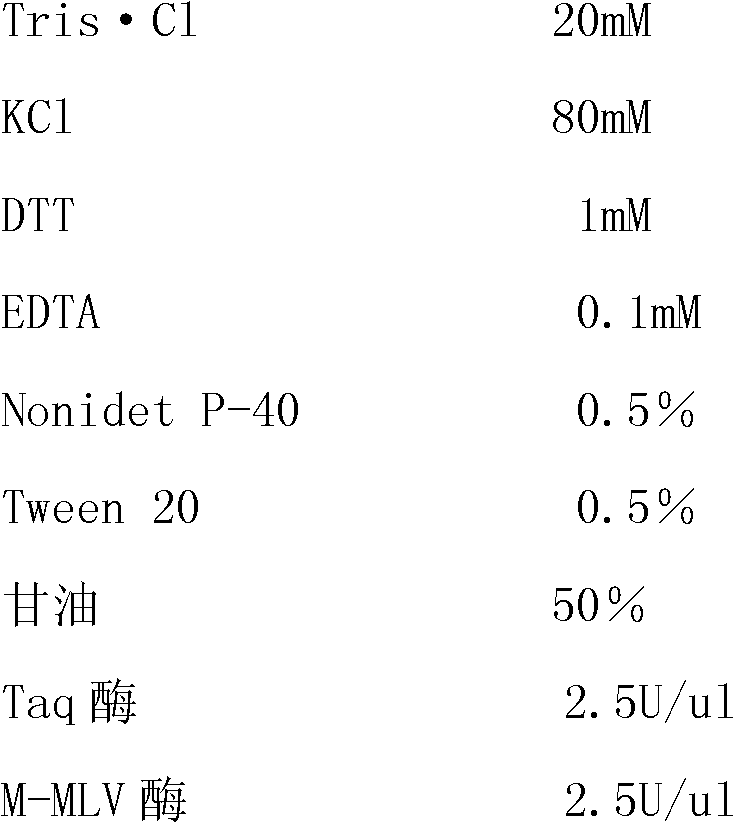
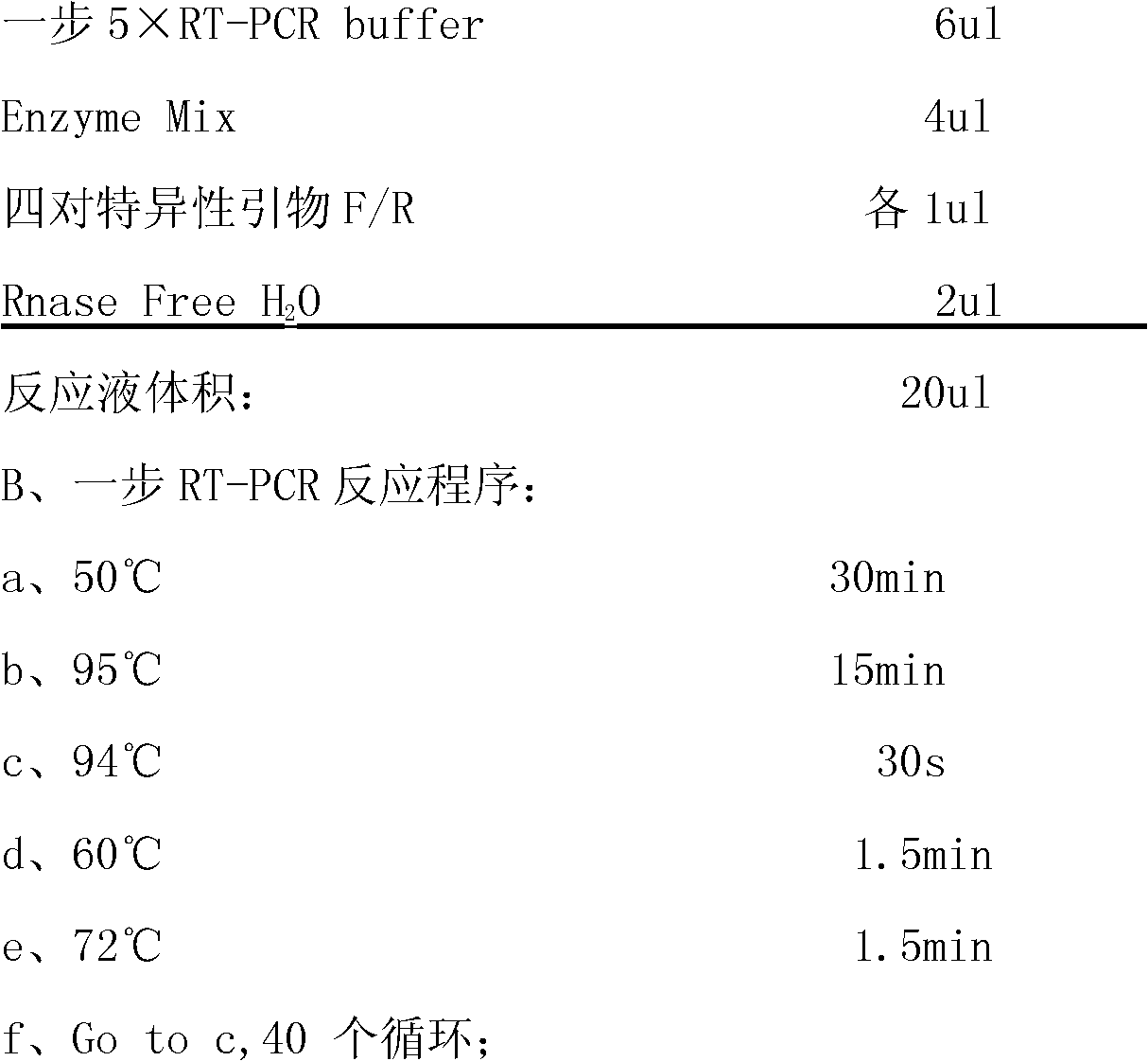
[0069] 1. The composition of the kit:
[0070]This kit includes one-step 5xRT-PCR Buffer, positive control, negative control, four pairs of specific primers and Enzyme Mix;
[0071] 2. Design specific primers for parainfluenza virus type 1
[0072] 1F: 5'-CCGGTAATTTCTCATACCTATG-3',
[0073] 1R: 5'-CCTTGGAGCGGAGTTGTTAAG-3';
[0074] Design of specific primers for parainfluenza virus type 2
[0075] 2F: 5'-AACAATCTGCTGCAGCATTT-3',
[0076] 2R: 5'-ATGTCAGACAATGGGCAAAT-3';
[0077] Design of specific primers for parainfluenza virus type 3
[0078] 3F: 5'-CTCGAGGTTGTCAGGATATAG-3',
[0079] 3R: 5'-CTTTGGGAGTTGAACACAGTT-3';
[0080] Design of specific primers for parainfluenza virus type 4
[0081] 4F: 5'-CTGAACGGT...
Embodiment 2
[0115] The above method was used to test the sputum samples of another 20 patients with suspected parainfluenza virus infection, and 8 samples were found to be positive for parainfluenza virus, including 2 cases of parainfluenza virus type 1 positive, 2 cases of parainfluenza virus type 2 positive, and parainfluenza virus positive in 2 cases. Three cases were positive for type 3 and one case was positive for parainfluenza virus type 4, the detection rate was 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com