Compositions and methods for the treatment of hepatitis c
A technology of hepatitis C virus and composition, applied in the direction of biochemical equipment and methods, chemical equipment and methods, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
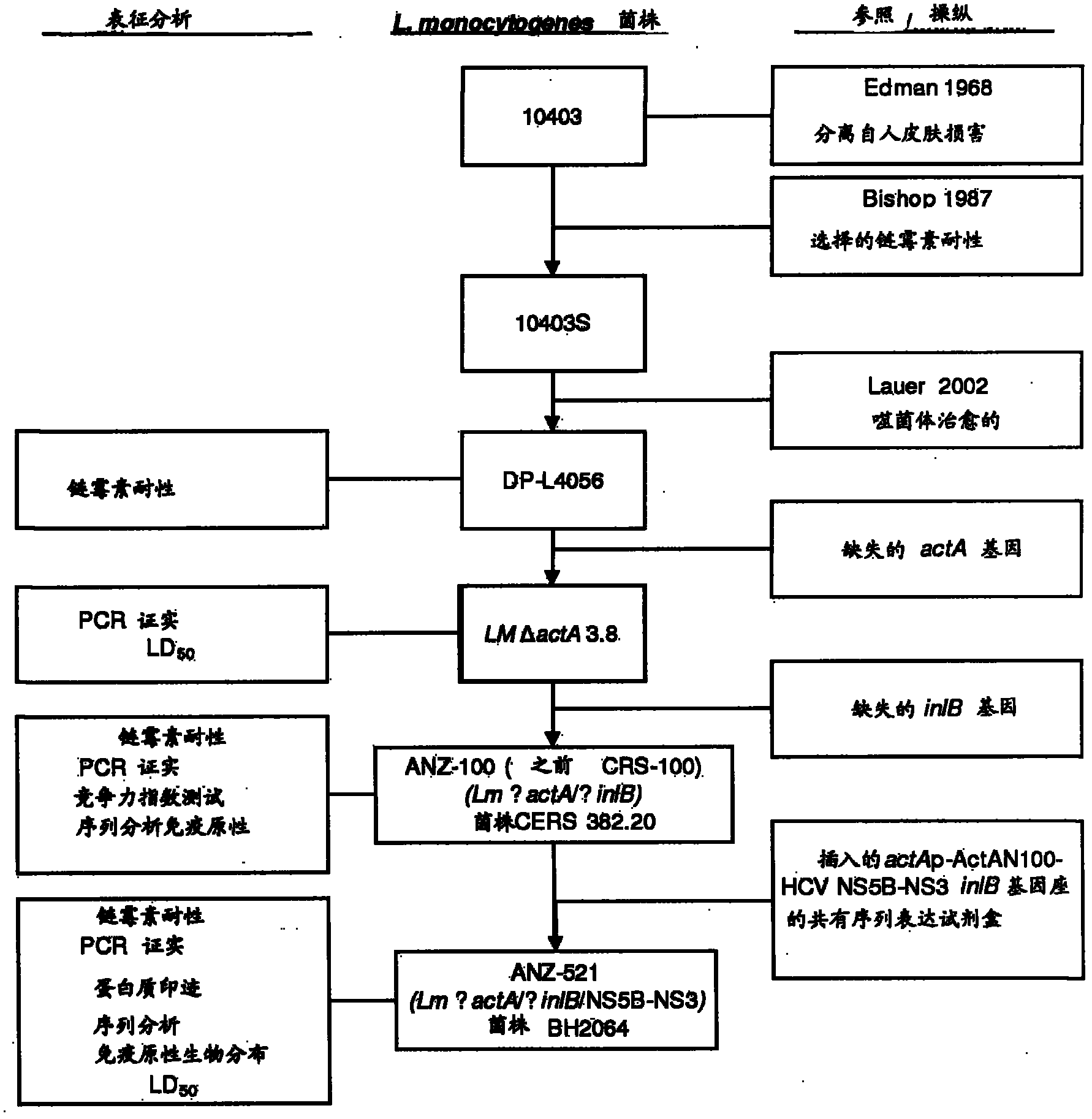
[0412] Example 1. Development of ANZ 100
[0413] The L. monocytogenes ANZ 100 vaccine platform strain was derived from L. monocytogenes strain DP L4056, a prophage-free derivative of L. monocytogenes strain 10403S, itself a streptomycin-resistant variant of wild-type L. monocytogenes strain 10403. Strain Lm 10403 was first isolated from human skin lesions (Edman 1968) and streptomycin-resistant strain 10403S was first described by Bishop and Hinrichs (Bishop 1987). Streptomycin resistance in 10403S was mapped to a single mutation at codon 56 of the ribosomal protein gene rpsL, in which a T to C nucleic acid substitution resulted in the insertion of an R (Lys) at position 56 instead The K(t(Arg) amino acid, the method used to isolate strain DP L4056 from Lm 10403S was previously described in detail (Lauer 2002).
[0414] Removal of actA and inlB pathogenic genes is accomplished by homologous recombination. The deletion of each gene requires three steps: (1) construction of a...
Embodiment 2
[0416] Example 2. Evaluation of HCV antigens
[0417] The Kyte-Doolittle Hydrophilic Chart is a widely used scale for depicting the hydrophobic properties of proteins. Hydrophobicity was calculated from the solvation enthalpy of individual amino acid residues and summed over a sliding window of 5 to 7 amino acids. A domain property with a value above 0 is hydrophobicity. Initial Kyte-Doolittle evaluations of HCV core, NS3 and NS5b antigens were used to identify regions less than or equal to peak hydrophobicity values derived from ActA-N100. Values greater than this may represent polypeptide sequences that do not express well in Listeria. The results are shown in Figure 7 .
[0418] Figure 8 Recombinant expression of Listeria antigens by various ActA-N100HCV antigen fusions is shown, as measured by Western blot. Individual HCV sequences (core sequences 1-190, 1-180, and 1-177; NS3 sequences 1-631, 1-484, 22-631, 22-484, 22-280, 172-484, 172-631, and 416 -631; and N...
Embodiment 3
[0425] Example 3. Development of ANZ 521
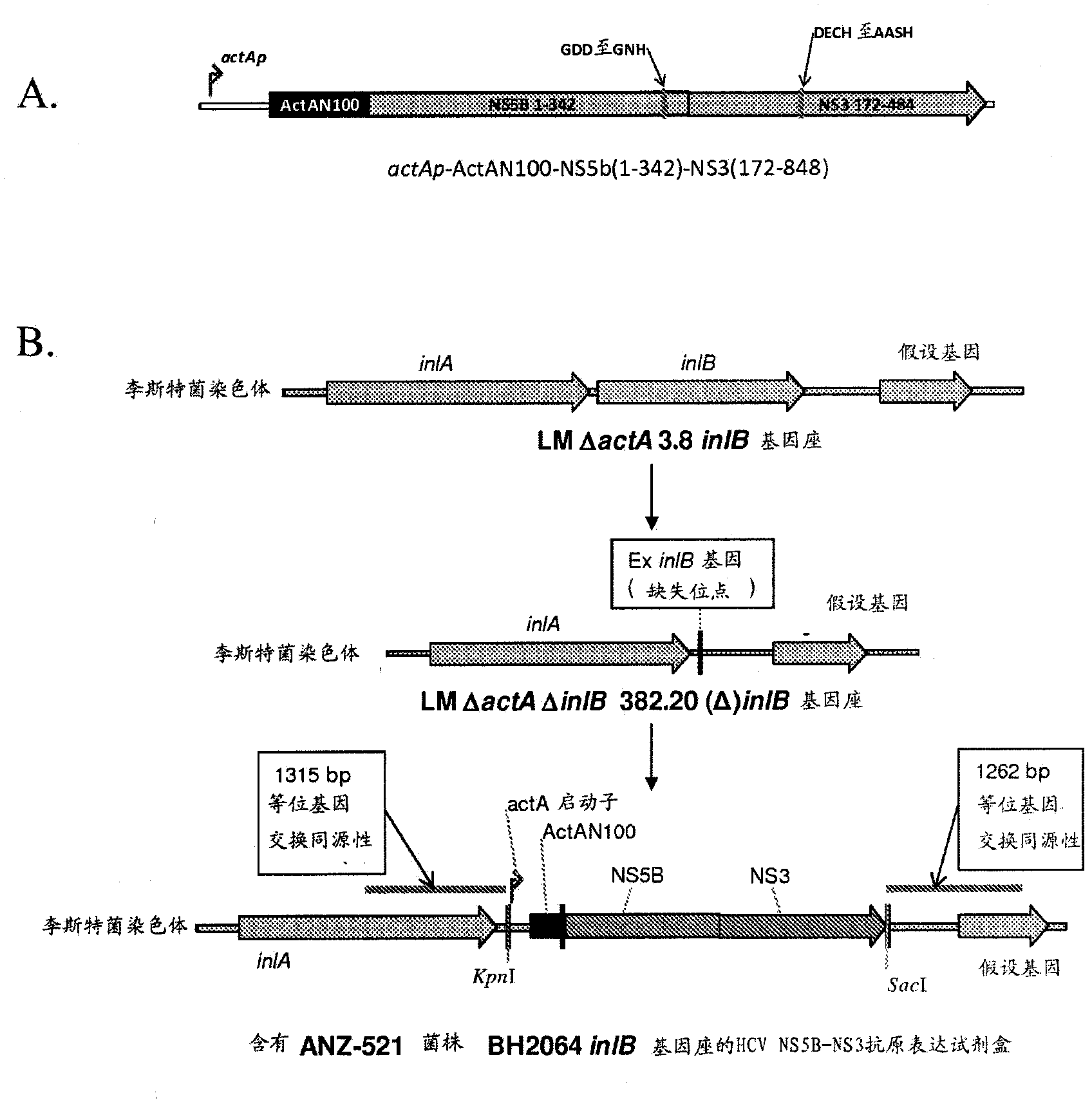
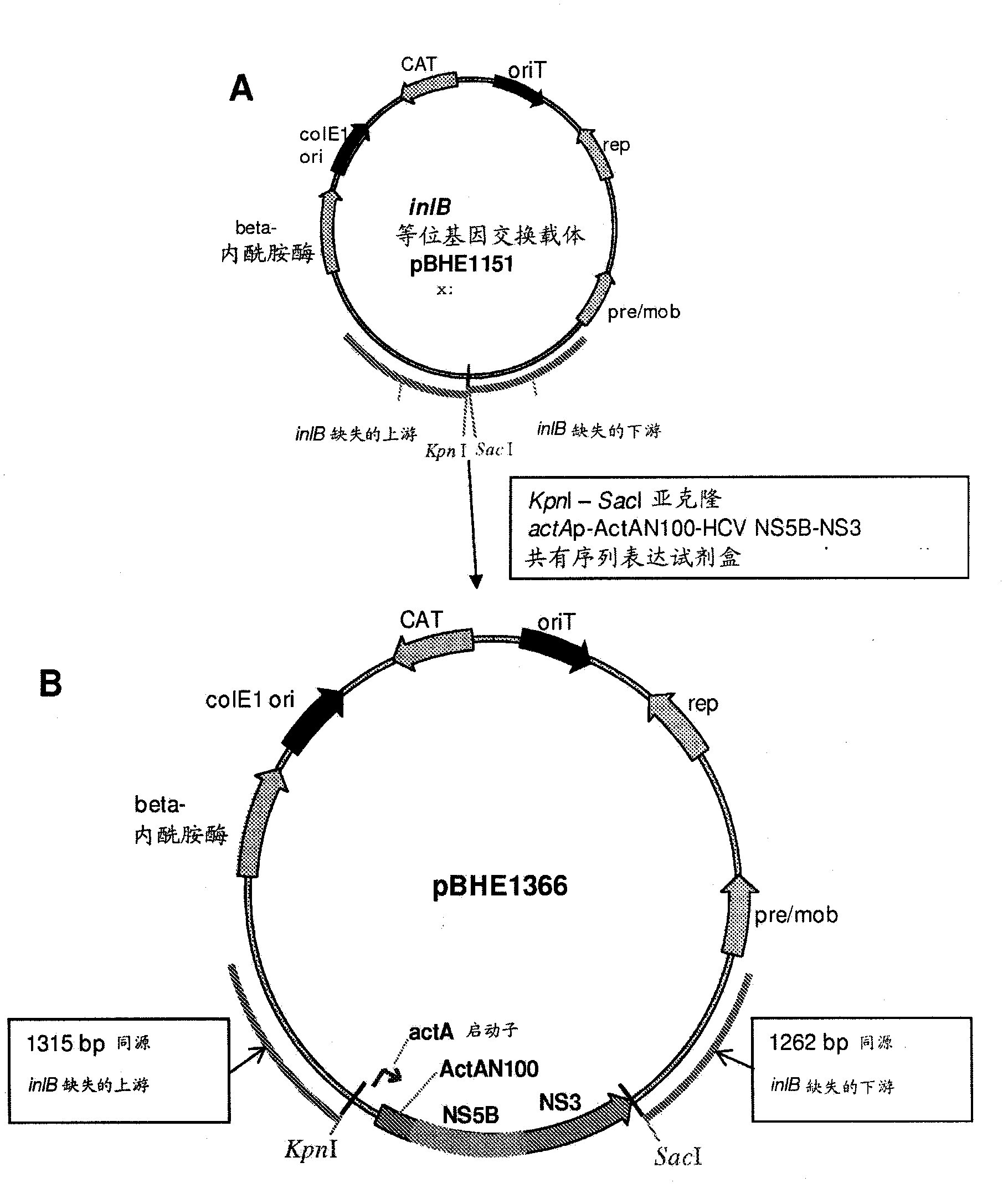
[0426] L. monocytogenes strain ANZ 521 is a Listeria vaccine strain based on the ANZ 100 vaccine platform. Figures showing the source and derivation of ANZ 521 are provided in figure 1 .
[0427] To develop ANZ 521, the antigen expression cassette ( figure 2 A) Constructed under the control of a bacterial promoter (L. monocytogenes ActA promoter) encoding portions of the HCV gene products NS5b and NS3. The expression cassette is stably integrated into the L. monocytogenes genome ( figure 2 B). The L. monocytogenes actA promoter was chosen because it is highly inducible in host cells. The HCV antigen comprising the NS5b and NS3 sequences is expressed as a single polypeptide fused to the amino-terminal 100 amino acids of the ActA protein ("ActA-N100"), which maximizes the expression and secretion of the HCV NS5B-NS3 fusion protein from bacteria that In the context of APCs infected in the inoculated host.
[0428] The expressed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com