Method for stable light focusing of fasudil hydrochloride and composition obtained using same
A technology of fasudil hydrochloride and a composition, which is applied in the directions of drug combination, pharmaceutical formula, drug delivery, etc., can solve the problems of inconvenient preparation process and safety inspection, hidden danger of safe medication, low permeability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 adds 0.05% photostabilizer methyl paraben on the basis of comparative example
[0020]
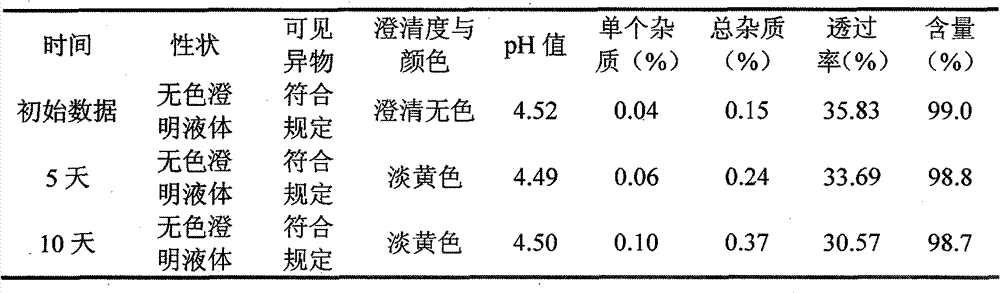
[0021] Detection method is equal to comparative example, and the obtained results are shown in Table 3
[0022] The various test results of the obtained composition of table 3 embodiment 1
[0023]
[0024] It can be seen from Table 3 that the total impurity content has no significant change, and the transmittance has no significant change, which shows that the composition is stable to light and does not need to be stored in the dark.
Embodiment 2
[0025] Embodiment 2 adds 0.15% light stabilizer on the basis of comparative example
[0026]
[0027] Detection method is equal to comparative example, and the obtained results are shown in Table 4:
[0028] The various detection results of the composition obtained in table 4 embodiment 2
[0029]
[0030] It can be seen from Table 4 that the total impurity content has no significant change, and the transmittance has no significant change, which shows that the composition is stable to light and does not need to be stored in the dark.
Embodiment 3
[0031] Embodiment 3 adds 0.25% photostabilizer methyl paraben on the basis of comparative example
[0032]
[0033] Detection method is equal to comparative example, and the obtained results are shown in Table 5
[0034] The various detection results of table 5 embodiment 3 gained compositions
[0035]
[0036] It can be seen from Table 5 that the total impurity content has no obvious change, and the transmittance has no obvious change, which shows that the composition is stable to light and does not need to be stored in the dark.
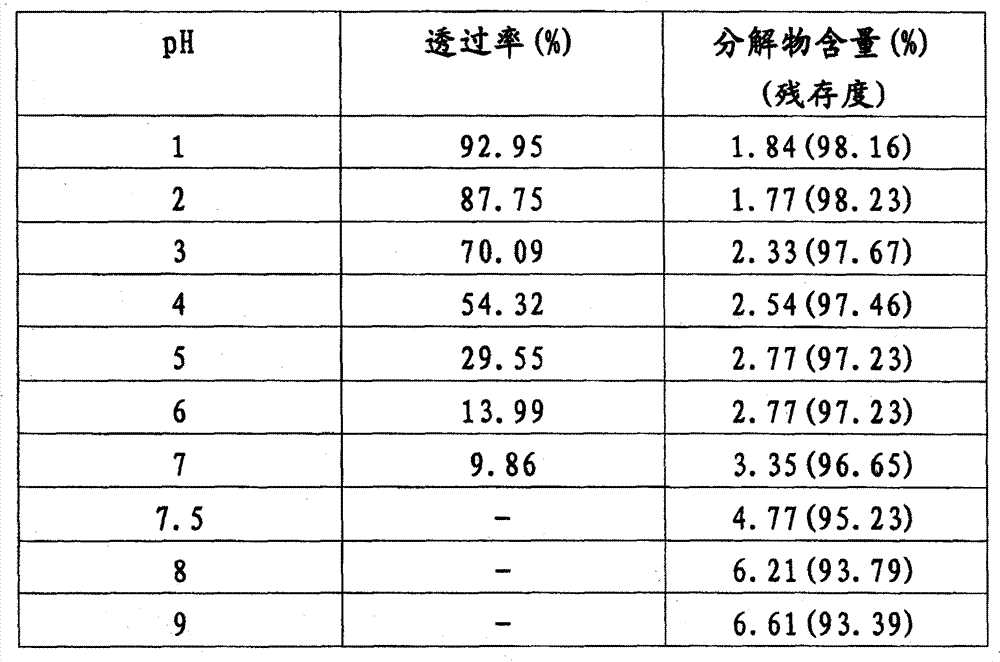
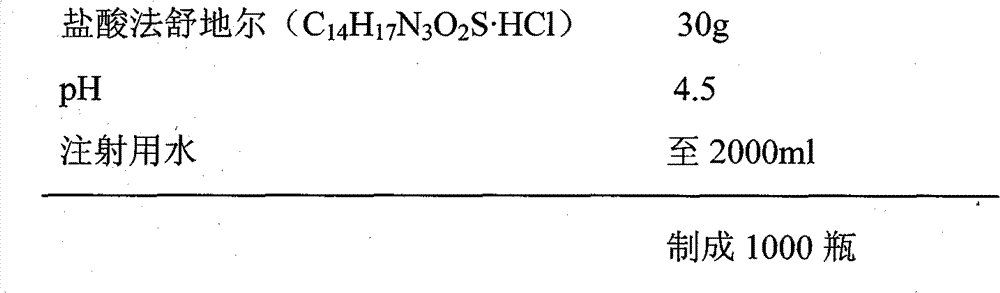
[0037] We have also investigated the stability under each pH value (regulating with hydrochloric acid or sodium hydroxide) when the content of sodium methylparaben (in methylparaben) is from 0.05% to 0.25%, the results show that it is all stable, and there is no Significant difference, enumerated again below the methyl paraben content is respectively 0.05, 0.15 and 0.25, and each inspection result that pH is measured when being 1 and 9 respe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com