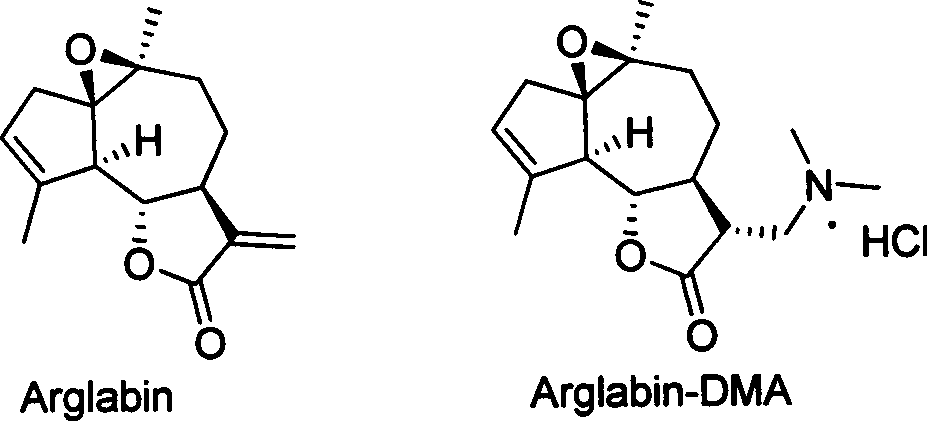
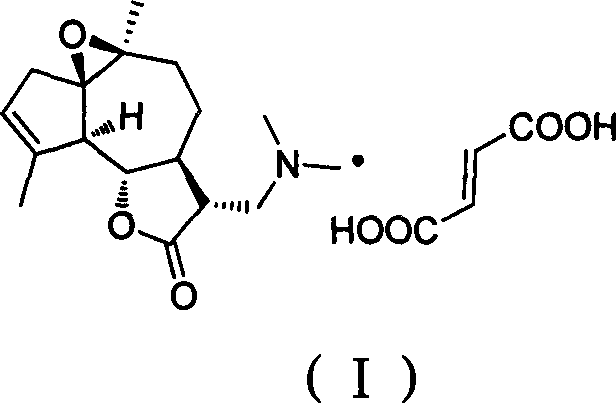
Agrarabine dimethylamine fumarate and its use in medicine preparation
A drug and application technology, applied in the field of agrarabine dimethylamine fumarate and its drug preparation, can solve the problems of large oral therapeutic window, low toxicity, small therapeutic window of Arglabin-DMA, etc., and achieve oral therapeutic window Large, low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Preparation of agrarabine dimethylamine fumarate
[0021] In the 50mL round bottom flask that is equipped with magnetic stirring, thermometer, reflux condenser, add fumaric acid (1.98g, 17mmol), agrarabine dimethylamine (4.95g, 17mmol), ethanol (20ml), mix homogeneously; Heat to 78°C and stir for 1 hour; concentrate under vacuum and remove the solvent to obtain a light yellow solid; mix the light yellow solid with ethyl acetate (150mL) evenly, heat from room temperature to 77°C, stir for beating, and cool naturally. After filtering, washing with ethyl acetate, and vacuum drying for 24 hours, 5.9 g of white solid powder was obtained with a yield of 85%. 1 H NMR (D 2 O, 400MHz) δ6.58(s, 2H), 5.57(br s, 1H), 2.66(m, 2H), 2.27(s, 6H), 2.24-2.03(m, 4H), 1.97(br s, 3H ), 1.84-1.48(m, 5H), 1.35(s, 3H).
Embodiment 2
[0022] Embodiment 2: Agrabine dimethylamine fumarate anticancer activity test
[0023] Match various cancer cells into 2×10 5 / mL cell suspension, add to 24-well round-bottomed cell culture plate, add woody hydrocarbon lactone derivatives or their salts respectively, 5 wells for each test concentration, place at 37°C, 5% CO 2 Cultivate under saturated humidity conditions for 18 hours, use MTT method to measure the absorbance (A) value at the wavelength of 570nm of the enzyme-linked detector, and calculate the inhibitory effect of the compound of the present invention on the test cancer cells.
[0024] Table 1 Inhibitory activity of coynelide and its derivatives on various cancer cells (IC 50 , μM)
[0025] cell
[0026] MCF-7
[0027] Among them, HL-60, HL-60 / A, K562, MCF-7, CNE-1, CNE-2, Du145, HT-29, A549, HepG-2, Ec9706, SGC7901, SW1116, A498, ASPC-1, HT -29, HeLa, GL15, B16F1, T24, SKOV3, SW579, and PC-3 represent acute leukemia cell lines, doxorubic...
Embodiment 3
[0028] Embodiment 3: Humidity comparison
[0029] Table 2 Humidity Comparison
[0030]
[0031] Referring to the Pharmacopoeia method, take 1.0g of each test sample, spread it on a glass watch glass with a thickness of about 1mm; place it in an environment with a temperature of 25°C and a relative humidity of 85% for 24 hours, and calculate the percentage of weight gain.
[0032] Agrabine dimethylamine fumarate is compared with Agrabine dimethylamine hydrochloride, and hygroscopicity is significantly improved, and is reduced to 0.25% (as shown in table 2) by 20.75%, has increased its stability scope , Broaden the scope of dosage form selection. Compared with the preparation process of agrabine dimethylamine fumarate and agrabine dimethylamine hydrochloride, the fumarate process is easy to operate, has low requirements for instruments and equipment, and relatively low cost. General experimental conditions It can meet the experimental scale from hundreds of grams to kilogra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com