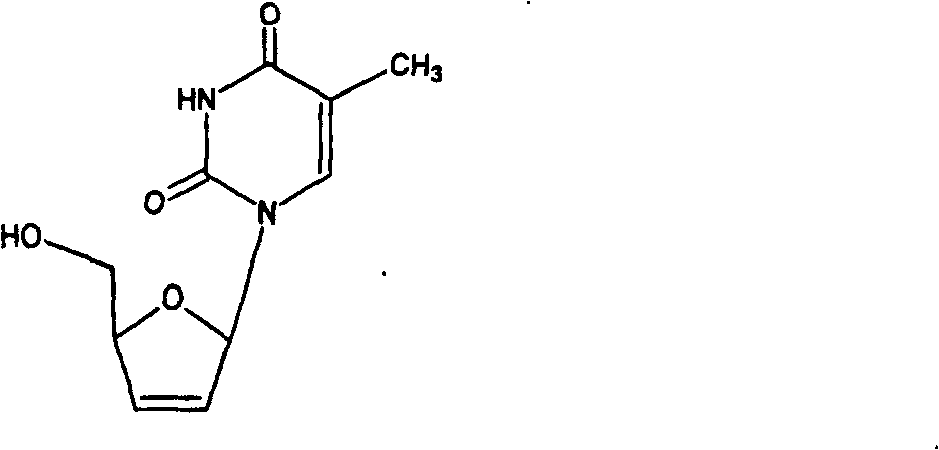
Oral preparation of quick releasing stavudine, and producing method
A technology for stavudine and immediate-release preparations, which can be used in pill delivery, antiviral agents, and pharmaceutical formulations, and can solve problems such as retention, difficulty in swallowing drugs, and discomfort
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] prescription:
[0084] Composition Weight percentage content
[0085] Stavudine 5%
[0086] cCMS-Na 6%
[0087] PVP (1% aqueous solution) 1%
[0088] Magnesium Stearate 0.5%
[0089] The rest is lactose added to 100%
[0090] Preparation:
[0091] First, the above-mentioned materials were crushed through a 100-mesh sieve, and stavudine, lactose, and cCMS-Na were mixed evenly, and 1% PVP aqueous solution was used as a binder to make a soft material, granulated through a 20-mesh sieve, and dried at 80°C. The 20-mesh granule is added with magnesium stearate, mixed evenly, and compressed into tablets.
Embodiment 2
[0093] prescription:
[0094] Composition Weight percentage content
[0095] Stavudine 5%
[0096] CMS-Na 6%
[0097] PVP (1% aqueous solution) 1%
[0098] Magnesium Stearate 0.5%
[0099] The rest is mannitol added to 100%
[0100] Preparation:
[0101] First crush the above materials through a 100-mesh sieve, mix stavudine, mannitol, and 60% CMS-Na evenly, use 1% PVP aqueous solution as a binder, make a soft material, pass through a 20-mesh sieve, and granulate at 80°C Dried, granulated in 20 meshes, added the remaining 40% CMS-Na and magnesium stearate, mixed evenly, and pressed into tablets.
Embodiment 3
[0103] Prescription weight percentage content
[0104] Stavudine 3.5%
[0105] Pregelatinized Starch 20%
[0106] Microcrystalline Cellulose 10%
[0107] LHPC 6%
[0108] PVP (1% aqueous solution) 1%
[0109] Magnesium Stearate 0.5%
[0110] The rest is lactose added to 100%
[0111] Preparation:
[0112]First, crush the above materials respectively through a 100-mesh sieve, mix stavudine, lactose, pregelatinized starch, microcrystalline cellulose, and LHPC evenly, use 1% PVP aqueous solution as a binder, make a soft material, and pass through a 20-mesh sieve Granulate, dry at 80°C, granulate into 20 meshes, add magnesium stearate, mix evenly, and compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com