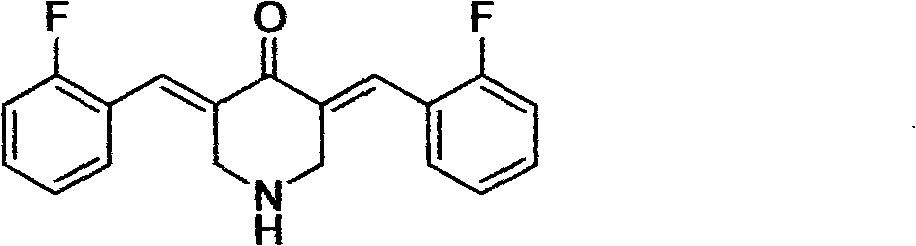
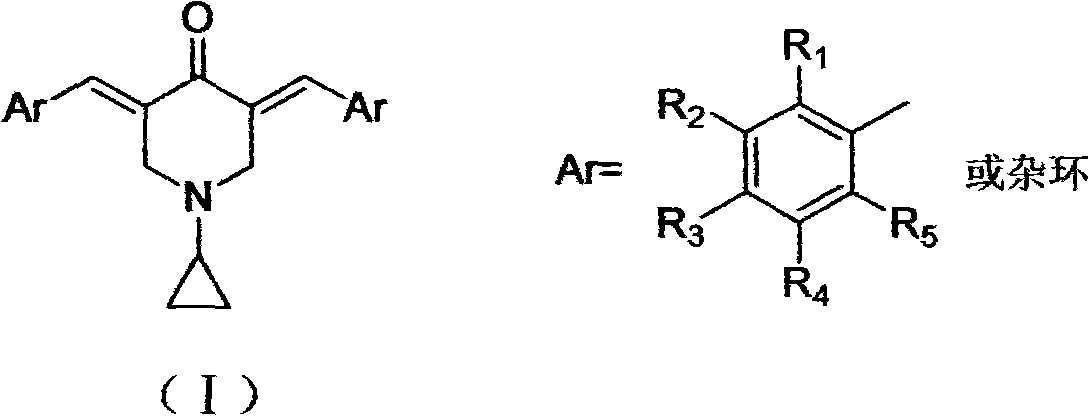
3,5-(E)-dibenzylidene-N-cyclopropyl piperidine-4-ketone and application of the 3,5-(E)-dibenzylidene-N-cyclopropyl piperidine-4-ketone in preparing antitumour drugs
A phenyl, pharmaceutical technology, applied in the direction of antineoplastic drugs, drug combinations, pharmaceutical formulations, etc., to achieve the effect of high efficiency, short time, simple separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1. When the aromatic aldehyde is 2,4,5-trimethoxybenzaldehyde, 3,5-(E)-bis(2,4,5-trimethoxybenzylidene)-N-ring Propylpiperidin-4-one (compound 1), orange-red powder, yield 60%, mp150~153℃, ESI-MS: 496.3(M+1); 1 H-NMR (CDCl 3 , ppm) δ: 8.02 (2H, s, -CH=), 6.82 (2H, s, Ar-H), 6.55 (2H, s, Ar-H), 4.63 (4H, s, -CH 2 -), 3.86 (18H, s, -OCH 3 ), 1.93(s, 1H, -N-CH(CH 2 ) 2 ), 0.44-0.39 (m, 4H, -CH (CH 2 ) 2 ).δ: 87.99, 154.18, 151.13, 142.54, 131.93, 116.36, 114.32, 109.17, 97.27, 56.86, 56.35, 56.21, 55.19, 37.42, 6.67
Embodiment 2
[0031]Example 2. When the aromatic aldehyde is 2-fluorobenzaldehyde, 3,5-(E)-bis(2-fluorobenzylidene)-N-cyclopropylpiperidin-4-one (compound 2) is obtained , is light yellow powder, yield 88%, mp169~172℃, ESI-MS: 352.3(M+1); 1 H-NMR (CDCl 3 , ppm) δ: 7.88 (2H, s, -CH=), 7.39~7.31 (4H, m, Ph-H), 7.22~7.11 (4H, m, Ph-H), 3.86 (s, 4H, -CH 2 -), 1.90(s, 1H, -N-CH(CH 2 ) 2 ), 0.47-0.40 (m, 4H, -CH (CH 2 ) 2 ). 13 C-NMR (100MHz, CDCl 3 , ppm) δ: 186.71, 162.22, 159.72, 135.09, 130.76, 129.25, 123.99, 123.25, 115.82, 54.98, 37.70, 6.77.
Embodiment 3
[0032] Example three, when the aromatic aldehyde is 3,4-dimethoxybenzaldehyde, 3,5-(E)-bis(3,4-dimethoxybenzylidene)-N-cyclopropyl is obtained Piperidin-4-one (compound 3), yellow powder, yield 66%, mp195~198℃, ESI-MS: 436.3(M+1); 1 H-NMR (CDCl 3 , ppm) δ: 7.75 (2H, s, -CH=), 7.06 (2H, d, J=8Hz, Ar-H), 6.97 (2H, s, Ar-H), 6.93 (2H, d, J= 8Hz, Ar-H), 4.01 (4H, s, -CH 2 -), 3.92 (12H, s, -OCH 3 ), 1.97(s, 1H, -N-CH(CH 2 ) 2 ), 0.53-0.48 (m, 4H, -CH (CH 2 ) 2 ). 13 C-NMR (100MHz, CDCl 3 , ppm) δ: 187.16, 150.01, 148.86, 136.26, 128.40, 124.04, 123.89, 114.04, 111.13, 55.99, 55.75, 55.12, 37.90, 6.73
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com