Method of decomposing n2o using a catalyst based on a cerium lanthanum oxide
A technology of catalysts and oxides, applied in the direction of metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, separation methods, etc., can solve problems such as insufficient catalyst stability, and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The method of preparation of this particular composition will now be described.
[0036] The method is characterized in that it comprises the following steps:
[0037] - forming a liquid medium comprising a cerium compound;
[0038] - heating the medium at a temperature of at least 100°C;
[0039] - separating the precipitate obtained in the preceding steps from the liquid medium, adding compounds of other rare earth metals (lanthanum and rare earth metals other than lanthanum and cerium) and forming another liquid medium;
[0040] - heating the medium thus obtained at a temperature of at least 100° C.;
[0041] - adjusting the reaction medium obtained in the above heating step to an alkaline pH;
[0042] - Isolation and calcination of the precipitate obtained in the preceding steps.
[0043] Thus, the first step of the method consists in forming a liquid medium comprising a cerium compound.
[0044] The liquid medium is usually water.
[0045] Preferably, the ceriu...
Embodiment 1
[0082] This example illustrates the N obtained on various catalysts after heat treatment at 950 °C under the above conditions 2 O conversion.
[0083] The catalysts were tested in the form of granules with a particle size between 0.5 mm and 1 mm. For each test, the weight of the catalyst was 10.5 g (i.e. the volume of the particles was 10 ml) and the hourly space velocity was 70000 h -1 .
[0084] The test conditions carried out in the laboratory are as follows:
[0085] Treated gas mixture contains 15% by volume water and 1000ppm N 2O, the rest is air. The water vapor content was regulated at a temperature of 60° C. by means of a stainless steel air humidifier.
[0086] Analysis of N by infrared at the outlet of the reactor 2 O. N is measured at a constant temperature equal to 850°C 2 O conversion.
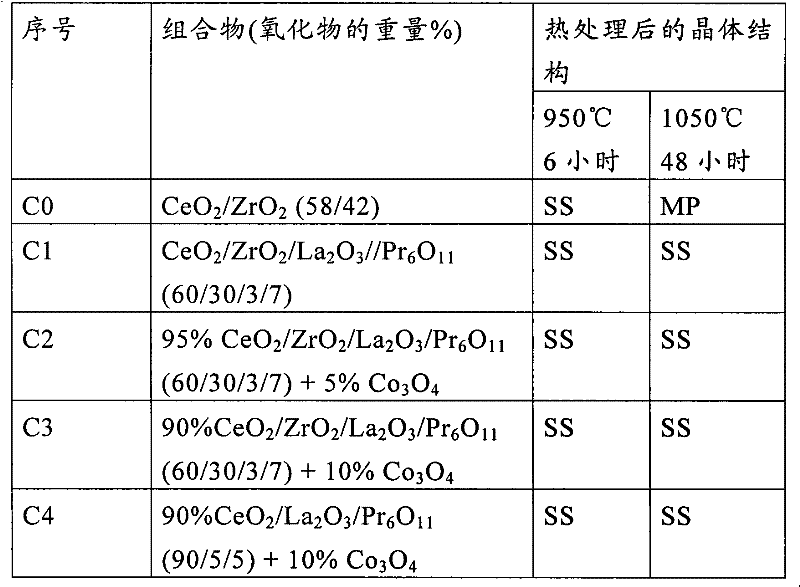
[0087] The results obtained are given in Table 2 below.
[0088] Table 2 Catalyst
[0089] Under the same experimental conditions, the conversion level (N 2 ...
Embodiment 2
[0091] This example illustrates the N 2 O conversion rate.
[0092] The shaping of the catalyst and its experimental conditions were the same as described in Example 1.
[0093] The results obtained are given in Table 3 below.
[0094] table 3
[0095] catalyst
[0096] Table 3 shows that after heat treatment at 1050 °C, the catalyst of the present invention obtained better N 2 O conversion rate. In addition, the decrease in the activity of the catalyst of the present invention is much smaller than that of the catalyst of the comparative example.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com