Reaction-type antimicrobial agent used for antimicrobial finishing of textiles
An antibacterial finishing agent and antibacterial finishing technology, which can be used in textiles and papermaking, fiber treatment, plant fibers, etc., and can solve the problems of lack of affinity for fibers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
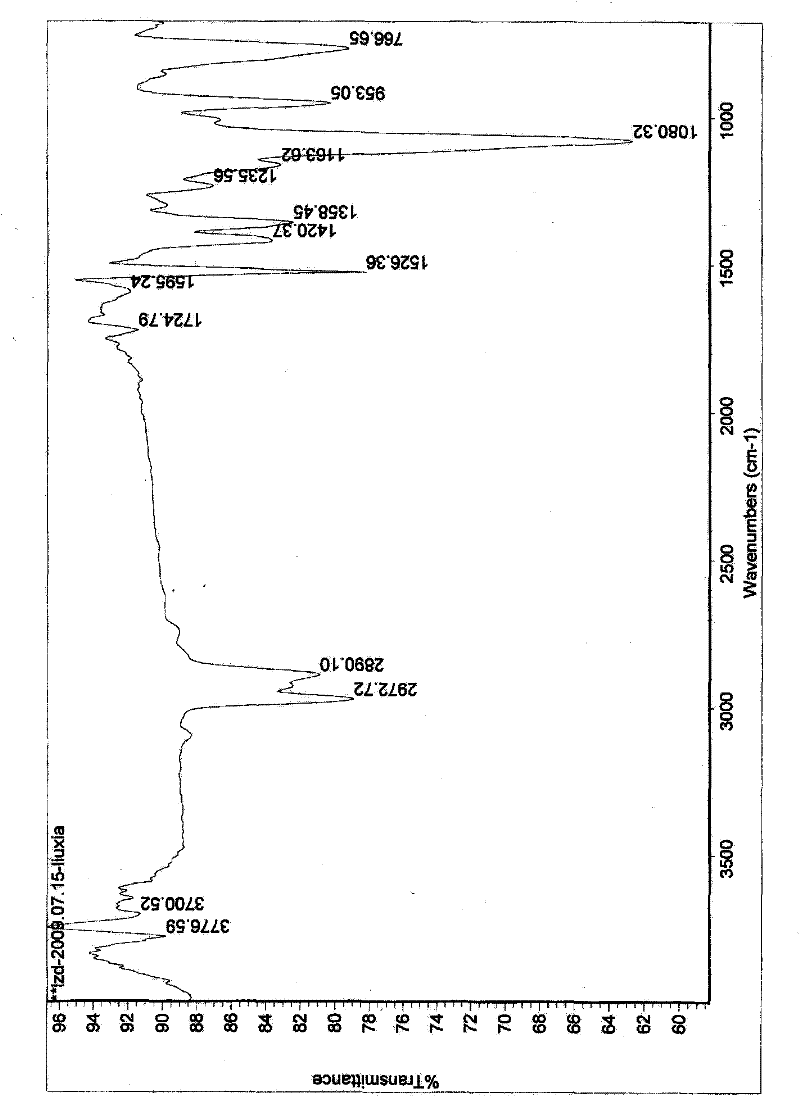
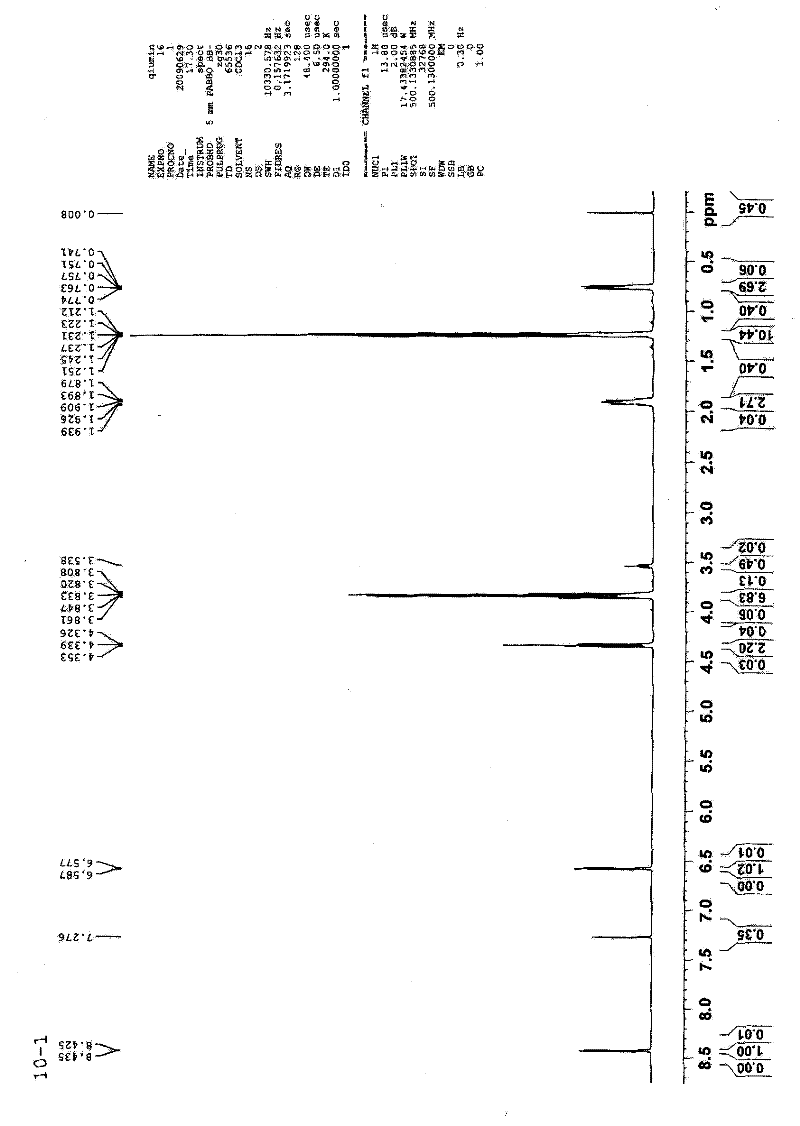
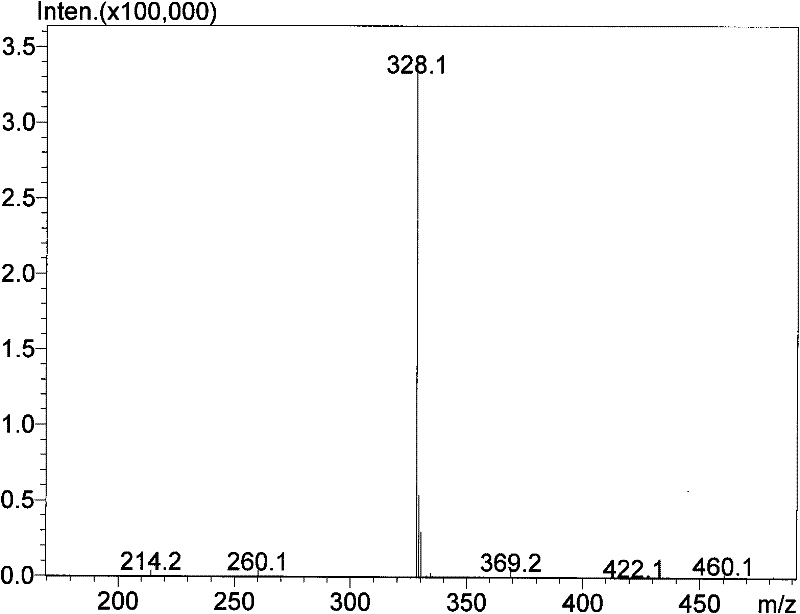
Image
Examples
Embodiment 1
[0056] Synthesis of N-triethoxysilylpropylisothiazolin-3-one
[0057] The air in the one-mouth eggplant-shaped flask under the ice-bath environment was exhausted with argon, and NaH, N, N-dimethylformamide (DMF) were added, and N of isothiazolin-3-one was added dropwise under electromagnetic stirring. N-dimethylformamide (DMF) solution, continue to stir until no bubbles are generated, and the whole process is protected by balloon argon; quickly add γ-chloropropyltriethoxysilane, and the molar ratio of the feed: isothiazolin-3-one: Sodium hydride: γ-chloropropyltriethoxysilane = 1: 1.2: 1.2; react in an ice bath for 30 minutes, transfer to an oil bath, heat up to 70-100°C, monitor the end point by TLC, react for about 1-5 hours and stop Heating; standing for cooling, rotary evaporation of DMF solvent, standing for cooling, adding ethyl acetate, suction filtering the precipitated salt, concentrating the filtrate, adding 80-100 mesh silica gel mixing material, and performing colu...
Embodiment 2
[0060] Synthesis of N-(4-bromobutyl)-isothiazolin-3-one
[0061] The air in the one-mouth eggplant-shaped flask under the ice-bath environment was exhausted with argon, and NaH, N, N-dimethylformamide (DMF) were added, and N of isothiazolin-3-one was added dropwise under electromagnetic stirring. N-dimethylformamide (DMF) solution, continue to stir until no bubbles are generated, and the whole balloon is protected by argon; slowly add the sodium salt of isothiazolin-3-one to 1,4- In dibromobutane, react at room temperature; after the dropwise addition, move it into an oil bath and slowly raise the temperature to 30-70°C, and monitor the end point by TLC; the reaction is complete in about 1-2h, stop heating, and let it cool down. Feeding molar ratio: isothiazolin-3-one: sodium hydride: dibromobutane=1:1.2:1.2. The DMF solvent was rotary evaporated, ethyl acetate was added, the precipitated salt was suction filtered, the filtrate was concentrated, and 80-100 mesh silica gel was...
Embodiment 3
[0064] Synthesis of N-triethoxysilylthiopropylbutylisothiazolin-3-one
[0065] Evacuate the air in the one-mouth eggplant-shaped bottle under the ice bath environment with argon, add NaH and absolute dry tetrahydrofuran (THF), slowly add γ-mercaptopropyltriethoxysilane dropwise under electromagnetic stirring, and the dropwise addition is completed Then continue to stir until no bubbles are generated; the whole process is protected by balloon argon. Add N-(4-bromobutyl)-isothiazolin-3-one, the molar ratio of N-(4-bromobutyl)-isothiazolin-3-one: γ-mercaptopropyltriethoxysilane : NaH=1:1.2:1.44; react in ice bath for 30min, transfer to an oil bath and slowly heat up to THF reflux temperature 66°C, TLC monitors the end point, and the reaction is complete in about 3h; stop heating, let stand to cool, and rotate to evaporate THF After adding ethyl acetate, the precipitated salt was suction filtered, and the filtrate was concentrated and mixed with 80-100 mesh silica gel for column ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com