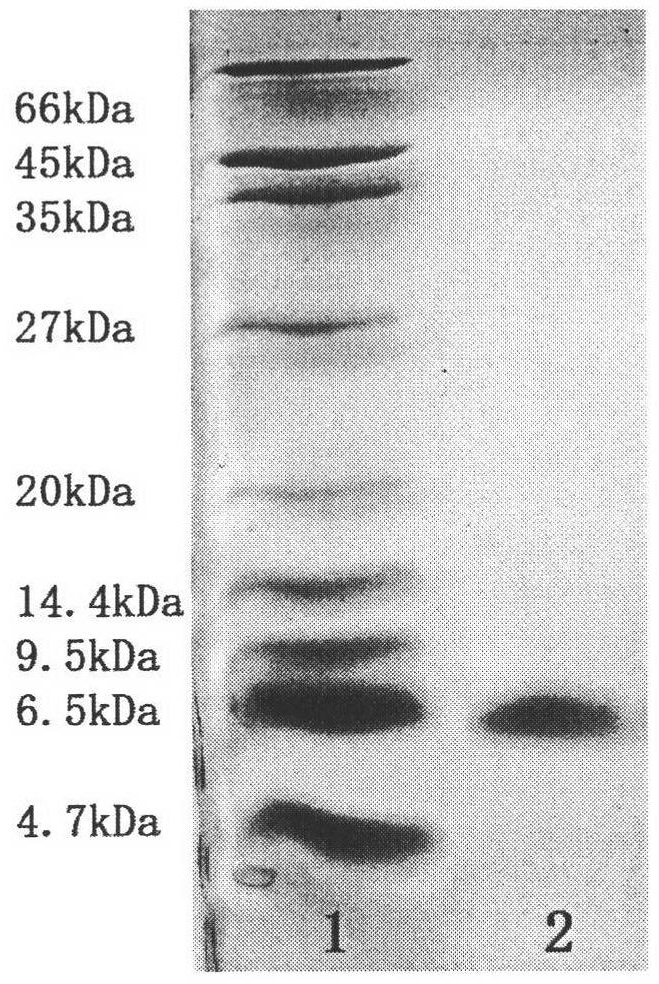
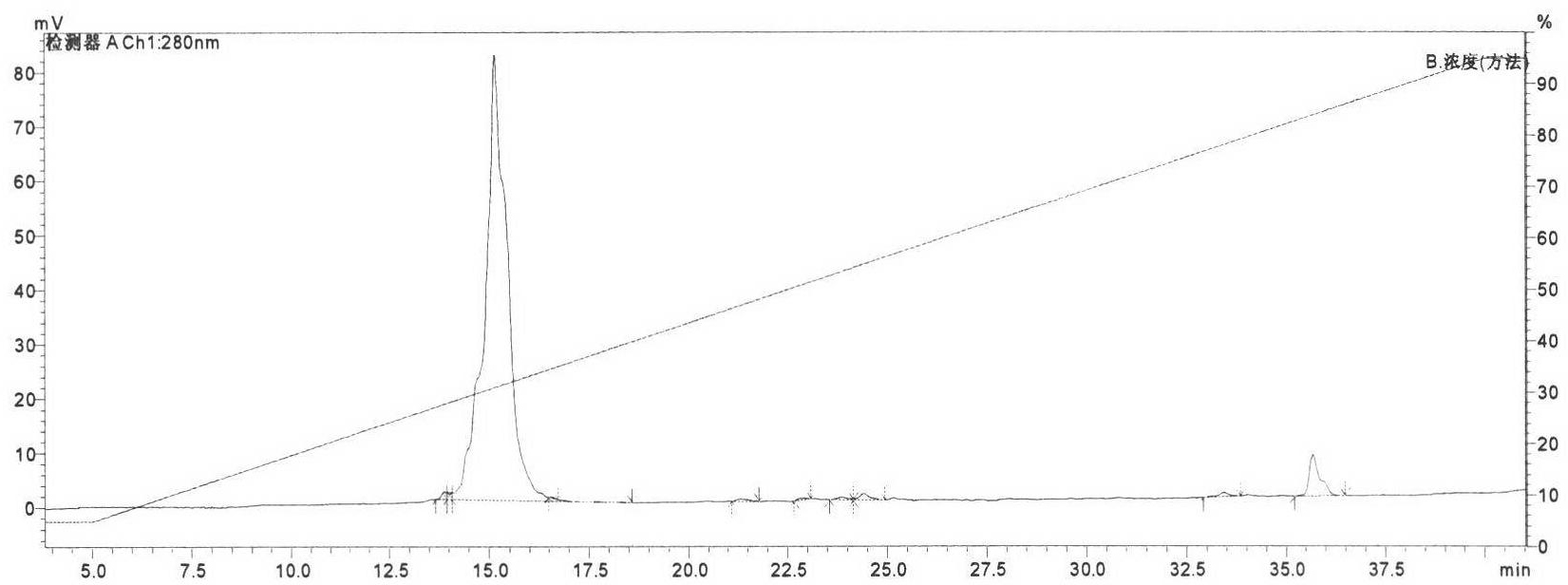
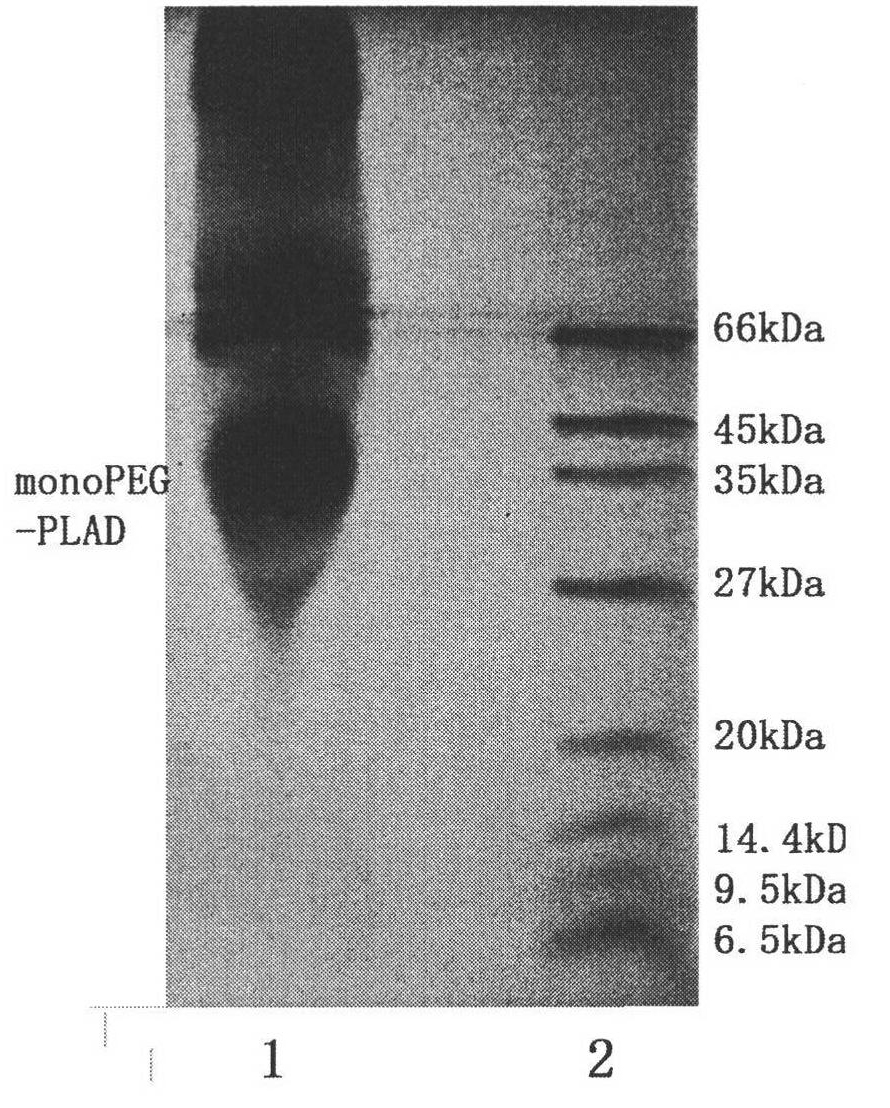
Molecular structure and application of tumor necrosis factor receptor 1 proligand assembly domain glycocluster
A tumor necrosis factor and conjugate technology, applied in the field of tumor necrosis factor receptor 1 pro-ligand assembly domain protein conjugates, can solve the problems of indistinguishability, increased incidence of lymphoma, inability to distinguish multiple TNFR pathways, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Coupling of mPEG-aldehyde to the N-terminal α-amino group of PLAD (SEQ ID NO: 1, Xaa=Leu)
[0031]Use 20mM acetic acid-sodium acetate buffer to configure PLAD into a protein solution of 10mg / ml, pH5.2, and add methoxypolyethylene glycol with a molecular weight of 20000Da using 20mM acetic acid-sodium acetate buffer Aldehyde (mPEG-ALD20000), so that the molar ratio of protein to PEG is 1:1, and sodium cyanoborocyanide at a final concentration of 20 mM is added to catalyze the reduction reaction. Leave to react at 4°C for 16 hours. After the reaction, dilute the reaction solution and adjust the pH value to neutral to stop the reaction. The reaction proceeds according to the following reaction formula:
[0032]
Embodiment 2
[0034] mPEG-maleimide coupled to Cys at the end of PLAD (SEQ ID NO: 2, Xaa=Cys)
[0035] Use 20mM Tris-HCl buffer to configure PLAD into a 6mg / ml, pH8.0 protein solution, add 2000Da methoxypolyethylene glycol maleyl with a molecular weight of 20mM Tris-HCl buffer imine (mPEG-MAL2000) such that the molar ratio of protein to PEG was 1:1. Leave to react at 4°C for more than 6 hours.
[0036] The reaction proceeds according to the following reaction formula:
[0037]
Embodiment 3
[0039] Coupling of mPEG-vinylsulfone to Cys at the end of PLAD (SEQ ID NO: 3, Xaa=Cys)
[0040] Use 20mM Tris-HCl buffer to configure PLAD into a 6mg / ml, pH 8.0 protein solution, add 5000Da methoxypolyethylene glycol vinyl sulfone with a molecular weight of 20mM Tris-HCl buffer (mPEG-VS5000) such that the molar ratio of protein to PEG was 1:2. Leave to react at 4°C for more than 6 hours. The reaction proceeds according to the following reaction formula:
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com