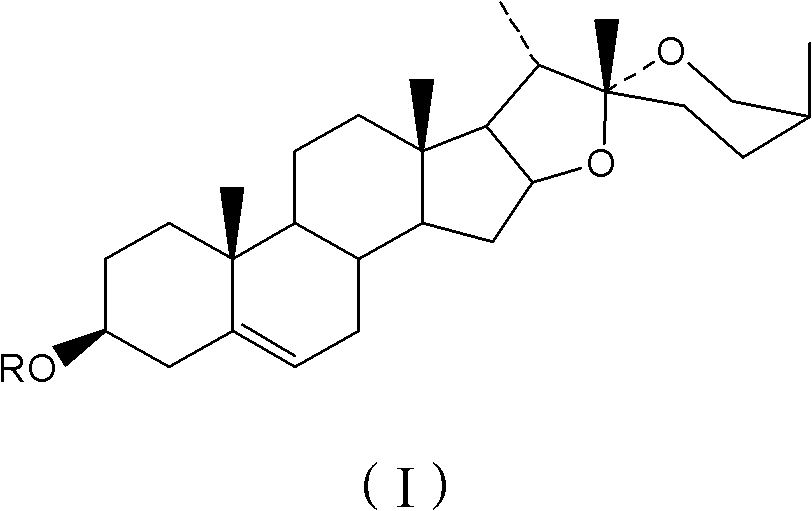
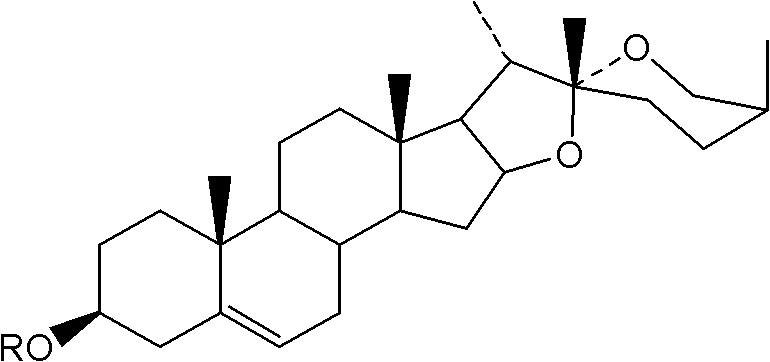
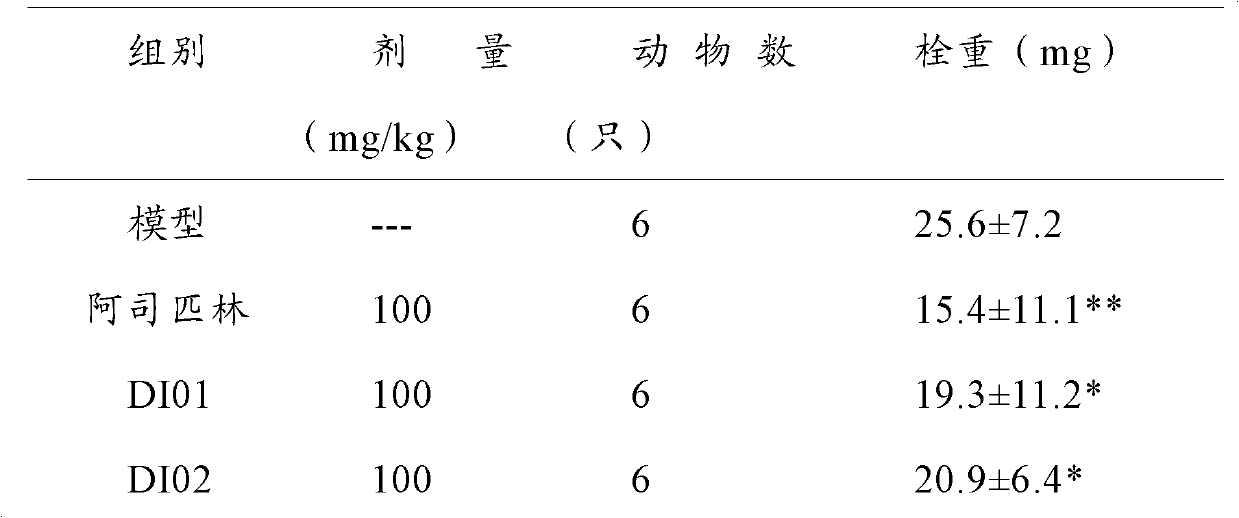
Steroid sapogenin derivatives and preparation method and application thereof
A technology of steroidal saponins and derivatives, applied in the field of medicine, can solve the problems of side effects such as inability to inhibit anti-platelet aggregation, intestinal reactions, changes in blood lipid levels, and bleeding tendency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0060] Example 13 Synthesis of β-benzoyldiosgenin
[0061] 10.4g (25mmol) of diosgenin was dissolved in 200mL pyridine in an ice-water bath, placed in a 500mL three-neck flask, 5.8mL (50mmol) of benzoyl chloride was added dropwise under electromagnetic stirring, and the reaction was allowed to rise to room temperature naturally for 24 hours. The reaction solution was poured into 1000mL H2O, with CH 2 C 12 Extract, combine the organic phases, wash with water, dilute hydrochloric acid, saturated NaHCO3 solution successively, dry over anhydrous MgSO4, filter, and evaporate the filtrate under reduced pressure (60°C) to obtain a slightly yellow product. Recrystallized with anhydrous EtOH, dried, weighed 10.6g, yield 81.8%.
[0062] Derivative spectral data:
[0063] ESI-MS: 519[M+H]+
[0064] NMR: 1 HNMR (400MHZ, CDCl 3 )δ: 0.85 (3H, s, H-18), 1.02 (3H, s, H-19), 1.15 (3H, d, J=7.2HZ, H-21), 0.69 (3H, d, J=6.0 HZ, H-27), 5.03 (1H, m, H-3), 5.38 (1H, d, J=4.8HZ, H-6), 4.54 (1...
Embodiment 23
[0065] Example 23 Preparation of β-diosgenin succinate monoester (DI 103) and its salt
[0066] 1) Preparation of 3β-diosgenin succinate monoester:
[0067] Weigh 4.14g (10mmol) of diosgenin and 2g (20mmol) of succinic anhydride, place in a 250mL three-necked flask, dissolve in 50mL pyridine, and dissolve. Place in a water bath equipped with an electromagnetic stirring device, react at an oil bath temperature of 80 °C, check the progress of the reaction by TLC (PE:EtOAc=3:1 v:v), and stop the reaction when it is found that the reactants are basically no longer converted. The reaction solution was evaporated under reduced pressure at 80°C to remove a large amount of pyridine. The solution was reddish brown. Add an appropriate amount of acetone and place it in the refrigerator for half a day. Slightly brownish yellow crystals precipitated. PE:EtOAc 5:1 as the mobile phase) method for separation and purification to obtain 0.79g of the product with a yield of 15.4%.
[0068] Der...
Embodiment 33
[0073] Example 33 Preparation of β-diosgenin sulfonate and salts thereof
[0074] 1) Preparation of 3β-diosgenin sulfonate
[0075] Add 1.2g of diosgenin and 40mL of freshly steamed pyridine into a 250mL three-necked bottle equipped with electric stirring and condenser, heat in a water bath at 35°C, start stirring to dissolve completely, and drop 4mL of chlorosulfur into the bottle under stirring Acid, after 20 minutes of dripping, the reaction was violent, producing a large amount of white smoke, recovered with lye, after the addition, continued to stir, TLC (CH2Cl2:MeOH:H2O=10:2:1HCOOH 1d) to detect the reaction progress. The reaction is relatively complete in about 30 minutes. Place in the refrigerator overnight, white crystals precipitate out, filter, wash with ice water, dry and weigh 0.3g, the yield is 21%.
[0076] Derivative spectral data:
[0077] ESI-MS: 493[M+H]+
[0078] NMR: 1 HNMR (400MHZ, CDCl 3 )δ: 0.81 (3H, s, H-18), 0.94 (3H, s, H-19), 1.13 (3H, d, J=7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com