Isoquinolinone derivatives
A technology for compounds and oxides, which can be used in drug combinations, active ingredients of heterocyclic compounds, digestive system, etc., and can solve problems such as unsatisfactory efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
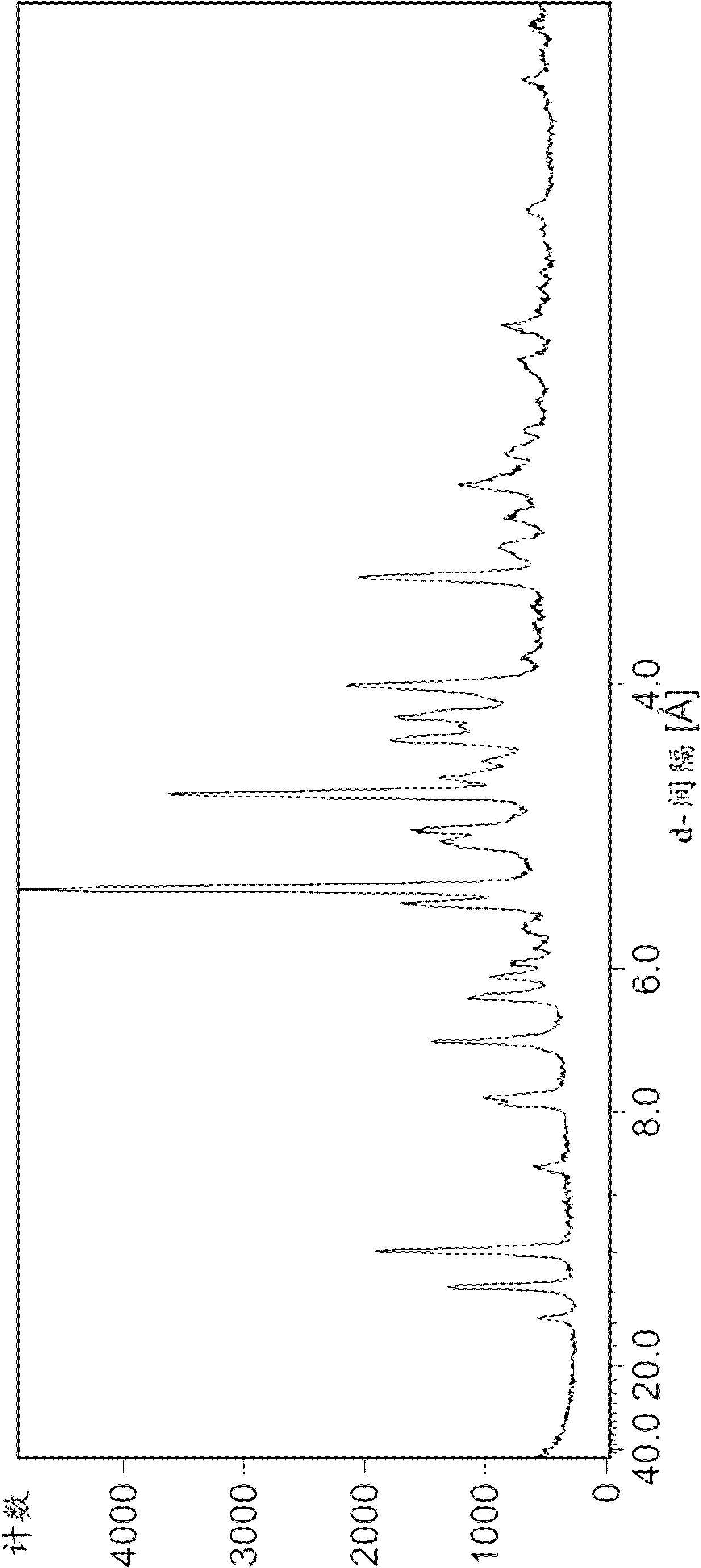
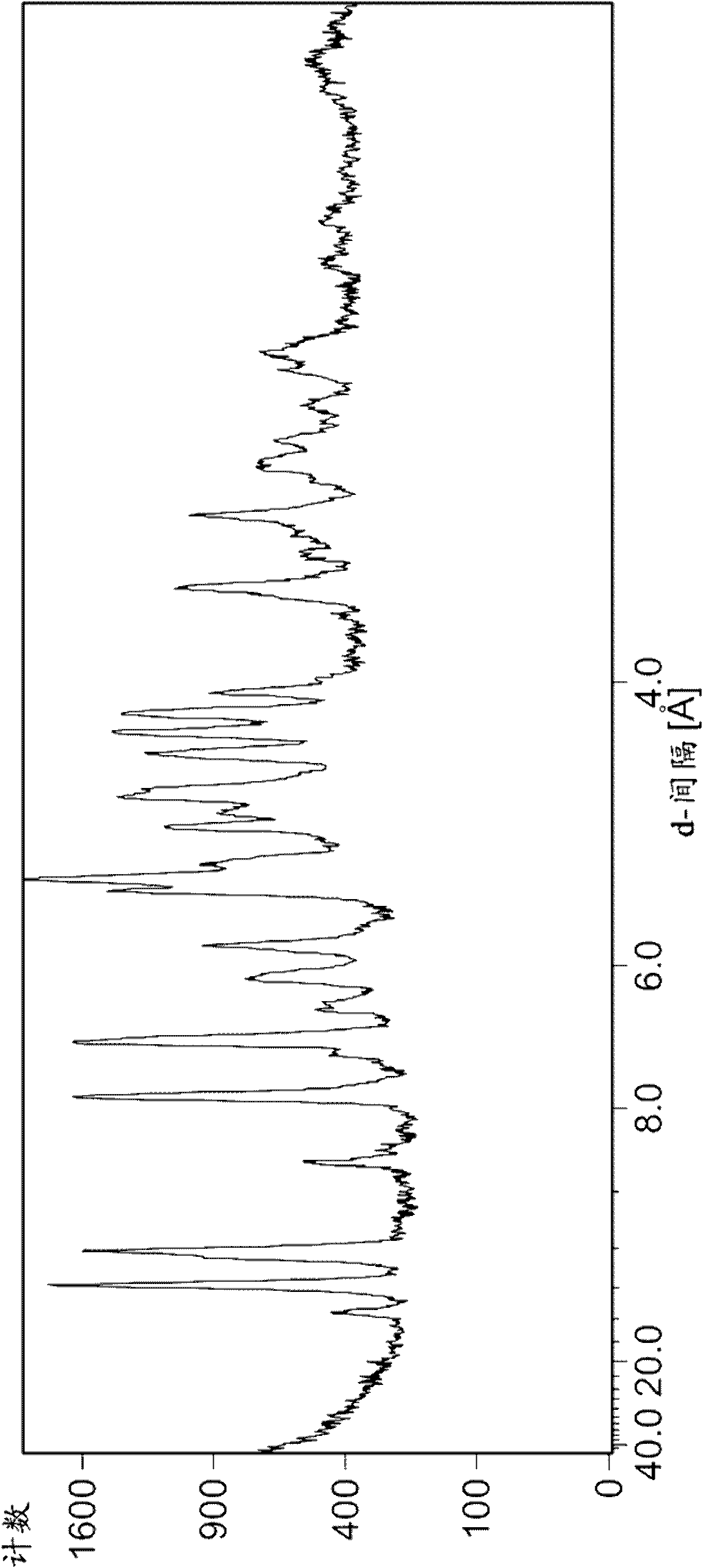
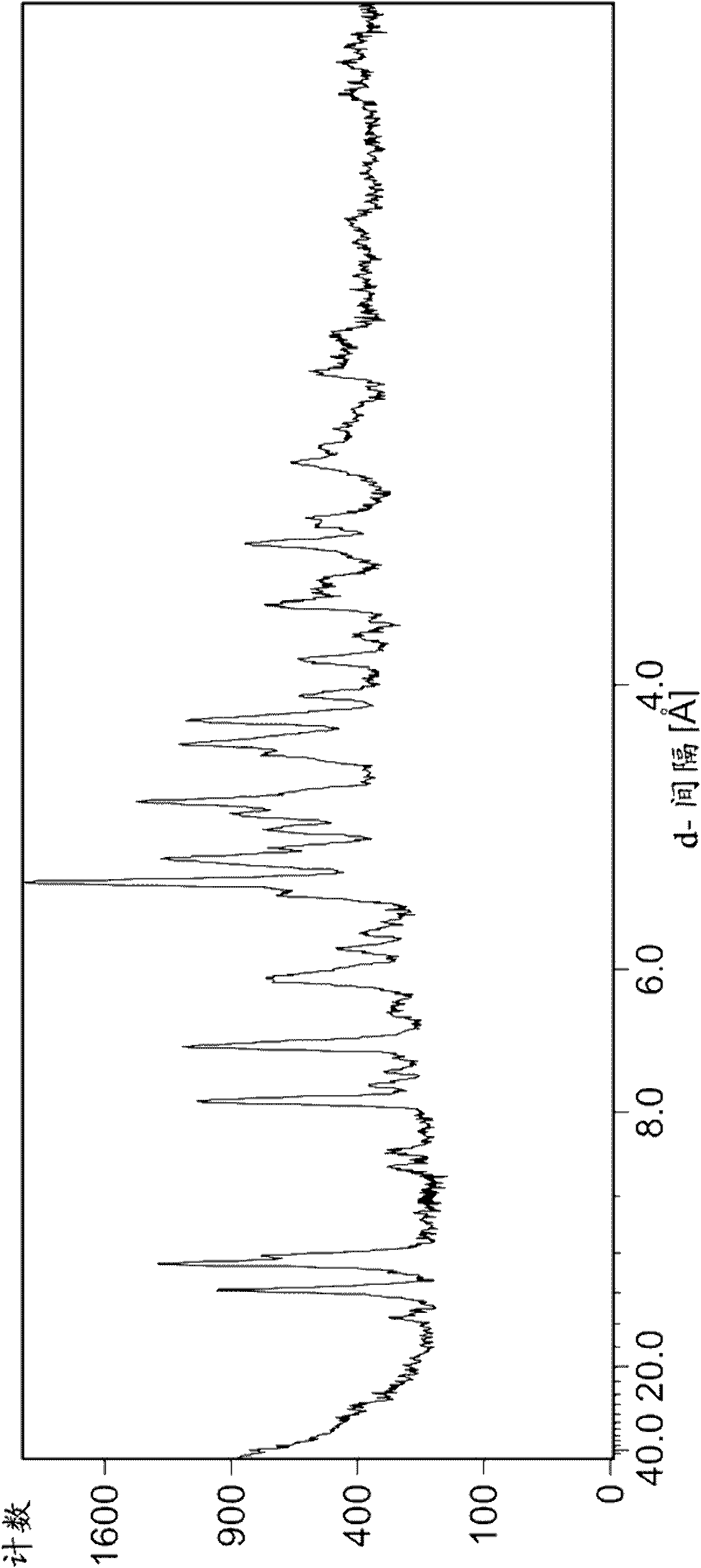
Image
Examples
Embodiment 1
[0505] N-cyclopropyl-3-fluoro-5-[2-(3-hydroxy-2,2-dimethylpropyl)-1-oxo-4-(piperazin-1-ylmethyl)-1 , 2-Dihydroisoquinolin-6-yl]-4-methylbenzamide
[0506]
[0507] a) 4-Bromo-2-[(E)-2-(dimethylamino)vinyl]benzonitrile
[0508] A solution of 4-bromo-2-methylbenzonitrile (1.0 g) dissolved in 1-tert-butoxy-N, N, N', N'-tetramethylmethylenediamine (2.11 ml) was heated at 140 Stir for 2 hours at °C (flask opened to evaporate tert-butanol). The reaction mixture was diluted with water (250ml) and extracted with ethyl acetate (350ml). dry (MgSO 4 ) organics, filtered and evaporated to give crude product. The crude product was triturated with isohexane overnight to give the subtitle compound (0.75 g) as a solid.
[0509] 1 H NMRδ(CDCl 3 )7.50(s, 1H), 7.34(s, 1H), 7.05-6.96(m, 2H), 5.29(d, 1H), 2.94(s, 6H)
[0510] b) 6-bromoisoquinolin-1(2H)-one
[0511] Under nitrogen atmosphere, 4-bromo-2-[(E)-2-(dimethylamino)vinyl]benzonitrile (Example 1a, 1.0 g) was dissolved in 33% hy...
Embodiment 2
[0530] N-cyclopropyl-3-fluoro-5-{2-(3-hydroxy-2,2-dimethylpropyl)-4-[(4-methyl-1,4-diazepane -1-yl)methyl]-1-oxo-1,2-dihydroisoquinolin-6-yl}-4-methylbenzamide
[0531]
[0532] a) 6-bromo-2-(3-hydroxyl-2,2-dimethylpropyl)isoquinolin-1(2H)-one
[0533] A solution of 6-bromoisoquinolin-1(2H)-one (Example 1b, 2.5 g) in NMP (20 ml) was treated with cesium carbonate (7.27 g) and 3-bromo-2,2 under nitrogen atmosphere. - Treated with dimethylpropan-1-ol (2.75ml). The resulting mixture was stirred at 100°C for 8 hours. More cesium carbonate (1 equiv), 3-bromo-2,2-dimethylpropan-1-ol (1 equiv) and water (5 mL) were added, and the resulting mixture was stirred at 130° C. for 10 hours. The incomplete reaction mixture was diluted with water and extracted with ethyl acetate. dry (MgSO 4 ) organics, filtered and evaporated to give crude product. The crude product was dissolved in NMP (20ml) and treated with cesium carbonate (7.27g) and 3-bromo-2,2-dimethylpropan-1-ol (2.75ml) and ...
Embodiment 3
[0547] N-cyclopropyl-3-fluoro-5-(4-(((3S)-3-(hydroxymethyl)piperazin-1-yl)methyl)-2-(3-hydroxypropyl)-1 -Oxo-1,2-dihydroisoquinolin-6-yl)-4-methylbenzamide
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com