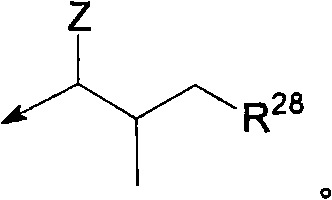
Pyrazinone derivatives and their use in the treatment of lung diseases
A technology of compounds and medicinal salts, applied in the field of pyrazinone derivatives, can solve problems such as unsatisfactory efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
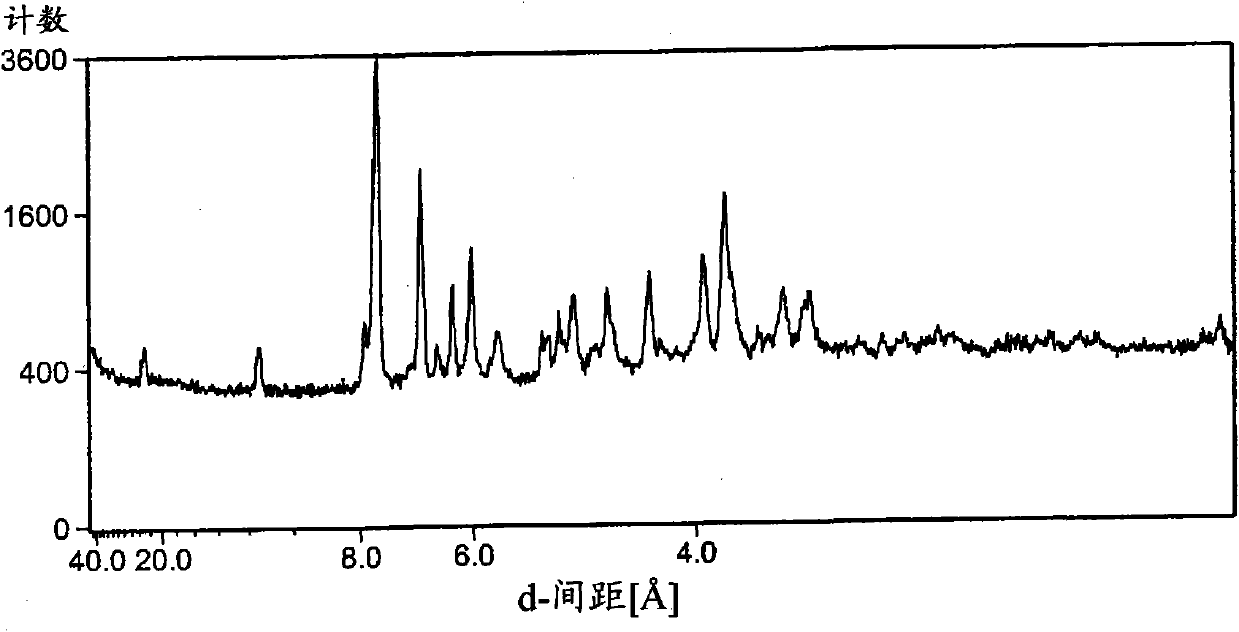
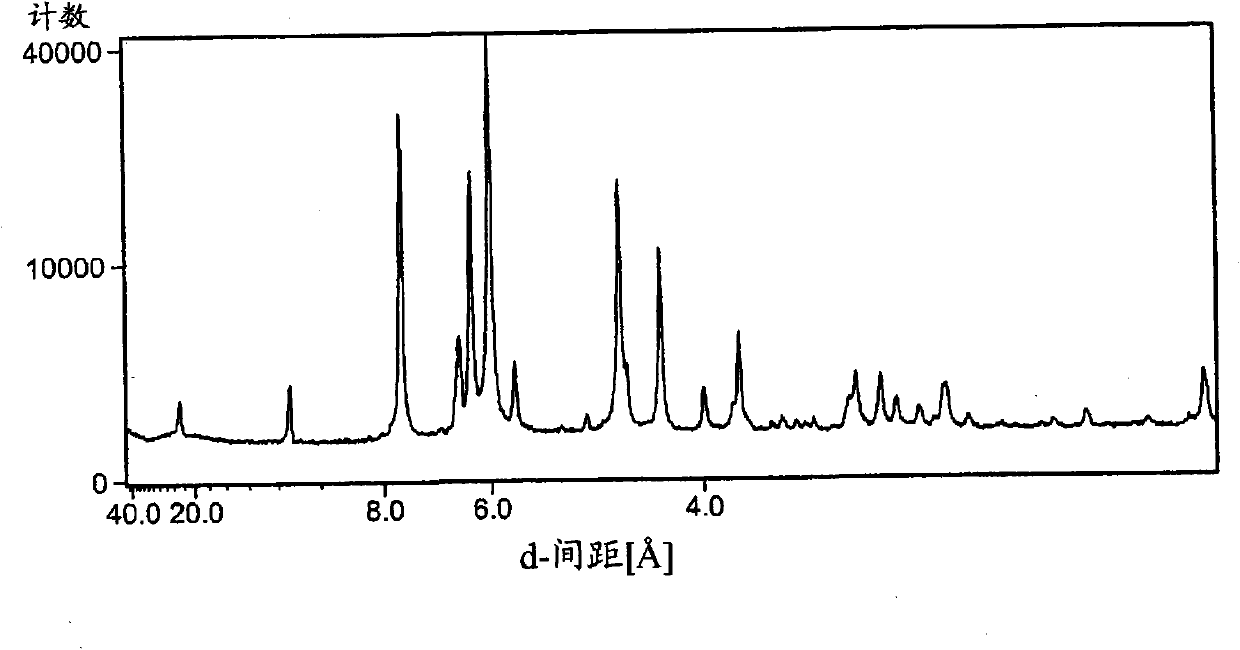
Image
Examples
Embodiment 1
[0707] N-cyclopropyl-3-[3-(2-fluoro-benzylamino)-2-oxo-2H-pyrazin-1-yl]-4-methyl-benzamide
[0708]
[0709] a) 3-[(cyanomethyl)amino]-4-methyl-benzoic acid methyl ester
[0710] To a stirred solution of methyl 3-amino-4-methyl-benzoate (10.0 g) in anhydrous tetrahydrofuran was added N,N-diisopropylethylamine (12.6 mL) followed by bromoacetonitrile (5.1 mL ). The reaction mixture was heated at reflux under nitrogen atmosphere while stirring for 12 h. Water was added, and the mixture was extracted with ethyl acetate. The pooled organics were washed with brine, dried (MgSO 4 ), filtration, and removal of the solvent under reduced pressure to afford the subtitle compound as a solid (12.4 g).
[0711] MS: APCI (+ve) 178 (M+H + ).
[0712] 1 H NMR (DMSO-d 6 , 300MHz) 7.31 (1H, dd), 7.23-7.17 (2H, m), 5.91 (1H, t), 4.33 (2H, d), 3.83 (3H, s), 2.17 (3H, s).
[0713] b) 3-(3,5-Dibromo-2-oxo-2H-pyrazin-1-yl)-4-methyl-benzoic acid methyl ester
[0714] To 3-[(cyanomethyl)...
Embodiment 2
[0723] N-cyclopropyl-4-methyl-3-[3-(4-methyl-piperazin-1-yl)-2-oxo-pyrazin-1(2H)-yl]-benzamide
Embodiment 3
[0725] N-cyclopropyl-3-[3-[[3-(dimethylamino)propyl]amino]-2-oxo-pyrazin-1(2H)-yl]-4-methyl-benzyl Amide
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com