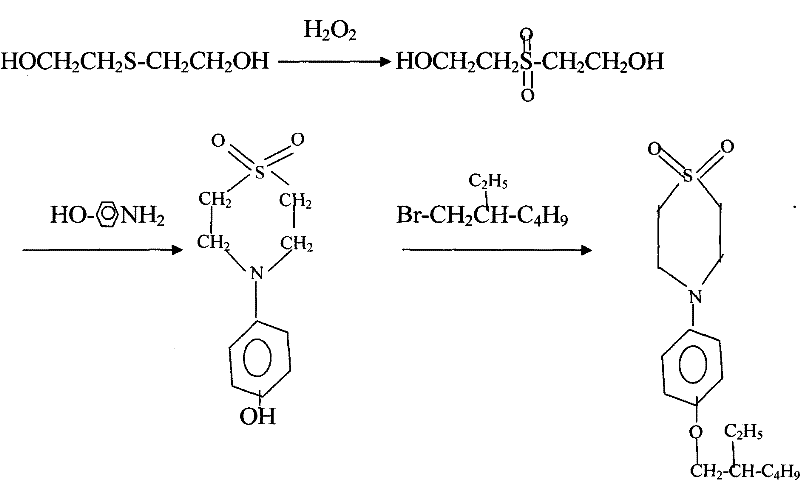
Preparation method of N-(4-(2-ethylhexyloxy) phenyl)-1, 1-dioxothiomorpholino
A technology of dioxythiomorpholine and ethylhexyloxy, which is applied in the field of photosensitive materials and heat-sensitive paper stabilizers, can solve the problems of large pollution, high production cost, and many reaction steps, so as to reduce energy consumption, The effect of high yield and low energy consumption index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
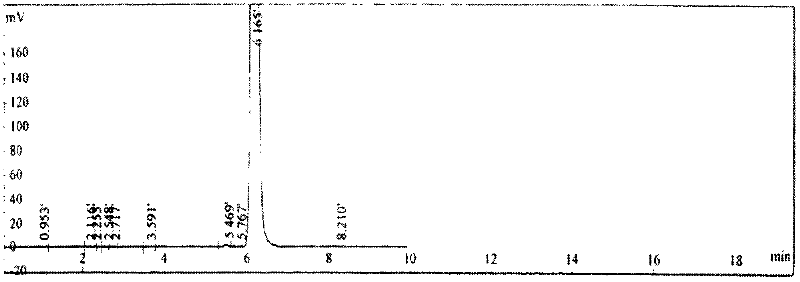
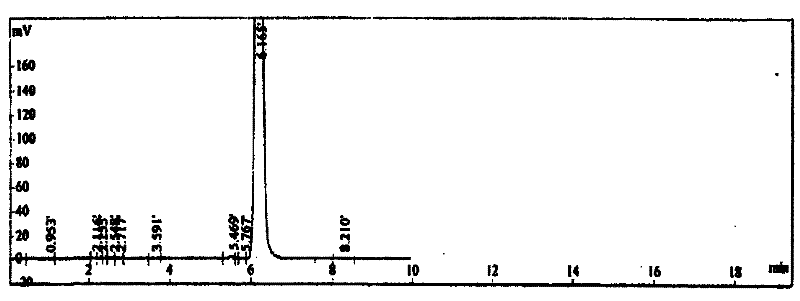
[0014] Example 1: Add 10 parts by weight of thiodiglycol and 10 parts by weight of water to the reactor, then add 1 part by weight of quaternary ammonium salt tetraethylammonium chloride as a catalyst, start stirring, and at a temperature of 60°C, Add 65 parts by weight of hydrogen peroxide dropwise to the reaction kettle, keep warm after dropping, and let it stand for 1 hour to generate bishydroxyethyl sulfone; then add 9 parts by weight of p-aminophenol, control the temperature at 40°C, perform condensation reaction for 3 hours, and then add 20 parts by weight of isooctane bromide, heated up to 100°C for further condensation reaction for 3 hours to obtain the product stock solution; pour n-heptane as the extraction solvent into the product stock solution, stir and let it stand, take the extract phase and evaporate the solvent to dryness Get the finished product. After calculation, the total yield reaches 86.8%; through gas chromatography analysis, the effective content of N-...
Embodiment 2
[0015] Example 2: Add 10 parts by weight of thiodiglycol and 10 parts by weight of water to the reactor, then add 1.5 parts by weight of quaternary ammonium salt tetraethylammonium chloride as a catalyst, start stirring, and at a temperature of 40°C, Add 70 parts by weight of hydrogen peroxide dropwise to the reaction kettle, keep warm after dropping, and let it stand for 1 hour to generate bishydroxyethyl sulfone; then add 10 parts by weight of p-aminophenol, control the temperature at 50°C, conduct condensation reaction for 3 hours, and then add 21 parts by weight of isooctane bromide, heated up to 80°C for further condensation reaction for 3 hours, to obtain the product stock solution; pour n-heptane as the extraction solvent into the product stock solution, stir and let it stand, take the extract phase and evaporate the solvent to dryness Get the finished product. After calculation, the total yield reaches 86.5%; through gas chromatography analysis, the effective content o...
Embodiment 3
[0016] Example 3: Add 10 parts by weight of thiodiglycol and 10 parts by weight of water to the reactor, then add 2 parts by weight of quaternary ammonium salt tetrabutylammonium bromide as a catalyst, start stirring, and at a temperature of 70°C, Add 75 parts by weight of hydrogen peroxide dropwise to the reaction kettle, keep warm after dropping, and let it stand for 1 hour to generate bishydroxyethyl sulfone; then add 10 parts by weight of p-aminophenol, control the temperature at 45°C, conduct condensation reaction for 3 hours, and then add 25 parts by weight of isooctane bromide, heated up to 95°C for further condensation reaction for 3 hours to obtain the product stock solution; pour n-heptane as the extraction solvent into the product stock solution, stir and let stand, take the extract phase and evaporate the solvent to dryness Get the finished product. After calculation, the total yield reaches 87.8%; through gas chromatography analysis, the effective content of N-(4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com