Application of solid acid catalyst to preparation of 2,5-dichloronitrobenzene
A technology of solid acid catalyst and dichloronitrobenzene, which is applied in the preparation of nitro compounds, organic chemistry, etc., can solve the problems of low product purity, incomplete reaction, and long reaction time, and achieve simplified reaction steps and complete reaction , the effect of high utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Put 1 mole part of p-toluenesulfonic acid and 1.25 mole parts of p-dichlorobenzene in a reaction kettle and stir and mix until uniform; the above materials are added dropwise at a temperature of 110 ° C. 1.3 molar parts of nitric acid were used for nitration reaction.
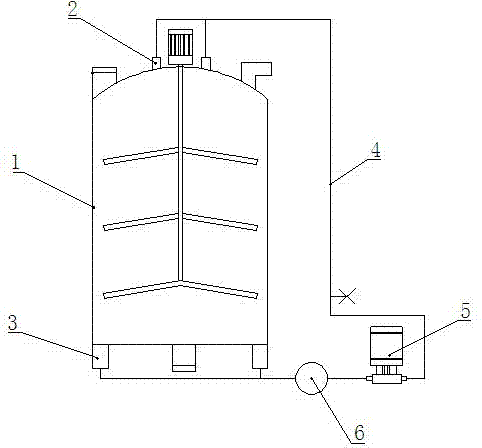
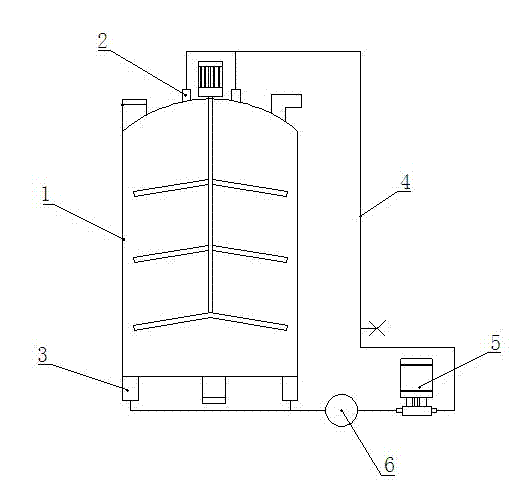
[0014] In the above nitration reaction, the material is an external circulation reaction, and the reaction kettle is specially made. The structure of the reactor is shown in the figure.
[0015] An outlet 2 is provided at the top of the kettle body 1 of the reaction kettle, an inlet 3 is arranged at the bottom, and a pipe 4 is used to connect the outlet 2 and the inlet 3, and a lifting pump 5 is arranged on the pipe 4 . When in use, the raw material enters the pipeline 4 from the outlet 2 at a flow rate of 5T / h through the lifting pump, and then returns to the kettle body 1 from the inlet 3, and the reciprocating cycle reacts for 6-8 hours to ensure that the material is fully reacted for 6-8 ho...
Embodiment 2
[0019] Put 0.8 mole parts of p-toluenesulfonic acid and 1.25 mole parts of p-dichlorobenzene in a reaction kettle and stir and mix them until they are uniform; 1.3 molar parts of nitric acid were used for nitration reaction.
[0020] In the above nitration reaction, the material is an external circulation reaction, and the reaction kettle is specially made. The structure of its reactor is identical with embodiment 1. The raw material enters the pipeline 4 through the lift pump from the outlet 2 at a flow rate of 5T / h, and the reciprocating cycle reacts for 4-6 hours to ensure that the material is fully reacted for 5 hours.
[0021] After the nitration reaction is completed, the temperature is controlled at 105° C. for 1-2 hours. After that, let it stand for 0.5 hours. After the temperature drops to 100°C, p-toluenesulfonic acid reaches the freezing point and forms a solid. The crude product of 2,5-dichloronitrobenzene is in a liquid state. Using the different freezing ...
Embodiment 3
[0024] Put 1.2 mole parts of p-toluenesulfonic acid and 1.25 mole parts of p-dichlorobenzene in a reaction kettle and stir and mix them until they are uniform; 1.3 molar parts of nitric acid were used for nitration reaction.
[0025] In the above nitration reaction, the material is an external circulation reaction, and the reaction kettle is specially made. The structure of its reactor is identical with embodiment 1. The raw material enters the pipeline 4 through the lift pump from the outlet 2 at a flow rate of 5T / h, and the reciprocating cycle reacts for 8-10 hours to ensure that the material is fully reacted for 8 hours.
[0026] After the nitration reaction is completed, the temperature is controlled at 110° C. for 1-2 hours. After that, let it stand for 0.5 hours. After the temperature drops to 100°C, p-toluenesulfonic acid reaches the freezing point and forms a solid. The crude product of 2,5-dichloronitrobenzene is in a liquid state. Using the different freezing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com