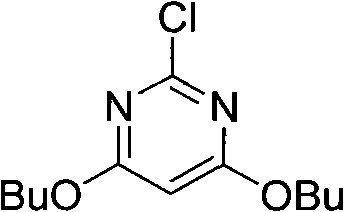
Synthetic method of 2-chloro-4,6-dibutoxypyrimidine
A technology of dibutoxypyrimidine and synthesis method, which is applied in the field of synthesis of 2-chloro-4,6-dibutoxypyrimidine, can solve the problems of easy decomposition and polymerization, unstable temperature, and cannot be industrialized, and achieves easy Recycling, easy recycling, reducing the effect of decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
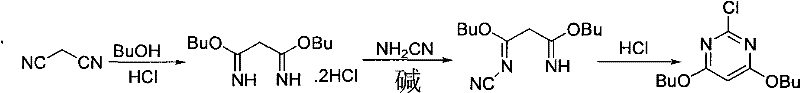
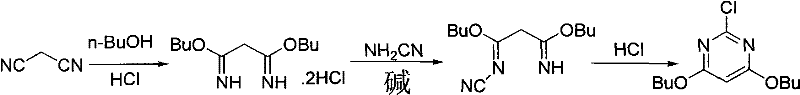
[0034] (1) Synthesis of Dibutoxypropanediamidine Hydrochloride (ADPX01)
[0035] Add 1000ml of ethyl acetate into a 2L four-neck flask, cool down in an ice-salt bath to 0-10°C; pass dry hydrogen chloride gas (292.0g, 8.0mol) for about 60 minutes, add malononitrile (132g, 2.0mol) of butanol (155g, 2.1mol)) solution, after the dropwise addition, keep warm at 10-15°C for 4 hours, filter, and dry the product at 60°C to obtain 406.0g of the product.
[0036] (2) Synthesis of 3-amino-3-butoxy-N-cyano-2-propionamidine (ADPX02)
[0037] Add NaHCO to a 2L four-neck flask 3 (181.0g, 2.2mol) and 1000ml of water, stirred, cooled in an ice-salt bath to below -0°C, added 50% NH 2 CN aqueous solution (214.0g, 2.55mol), add dibutoxypropanediamidine hydrochloride (ADPX01) (406.0g,), monitor the feeding rate through pH, and control the process pH at 9-10; after the reaction, keep warm at about 20°C Suction filtration for 4 hours; the filter cake was rinsed with water, drained and then drie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com