Tumor chemotherapeutic medicinal preparation and preparation method thereof
A chemotherapeutic drug, chemotherapeutic drug technology, applied in the directions of antitumor drugs, drug combination, drug delivery, etc., can solve the problems of increasing the toxic and side effects of nano-carrier chemotherapeutic drugs on the body, the toxic and side effects of biological organisms, and unfavorable clinical promotion and application. The effect of exerting drug efficacy, reducing drug resistance, and improving drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
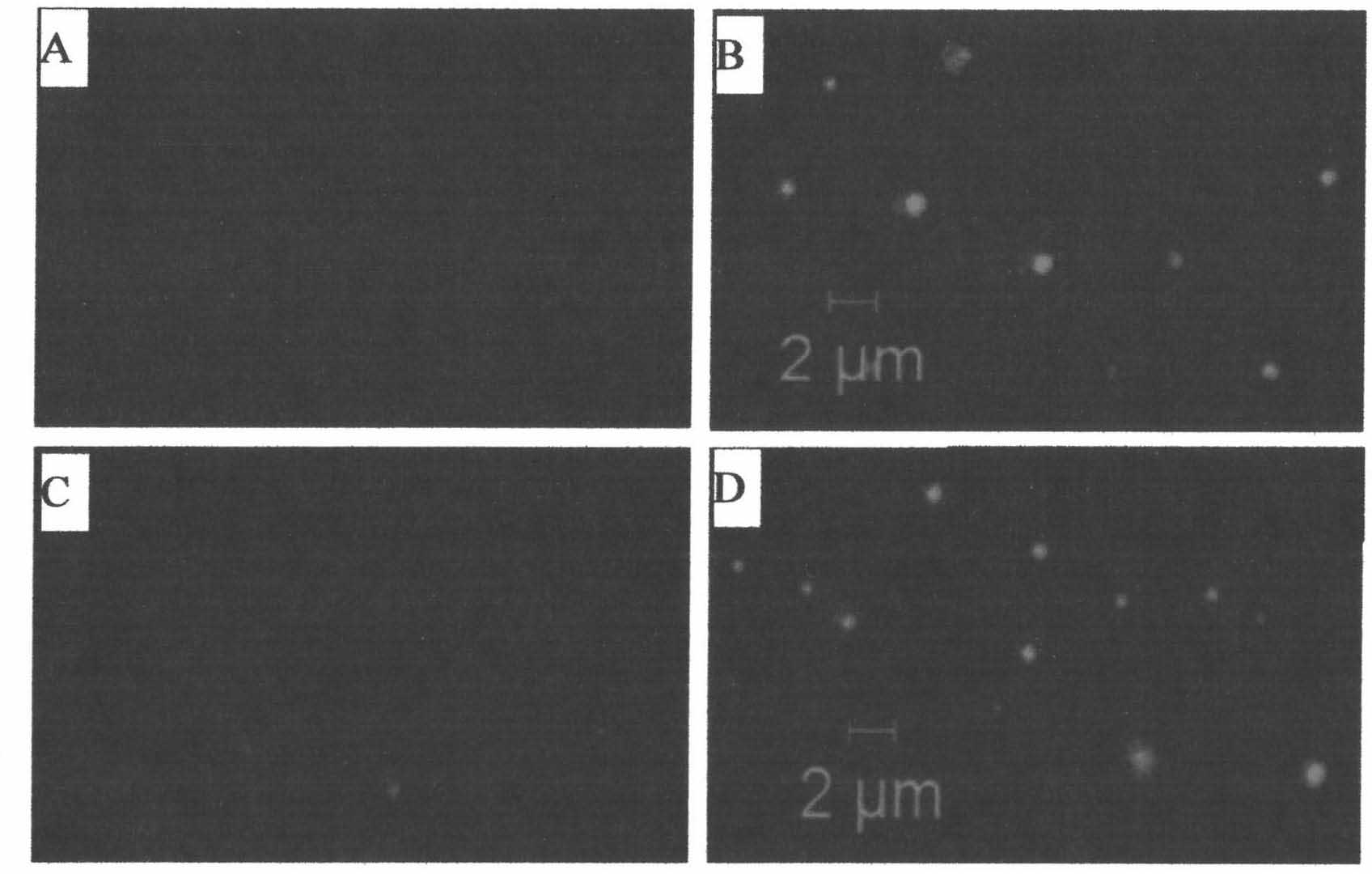
[0044] Example 1: Using chemotherapeutic drugs to induce apoptosis of mouse liver cancer cells and human ovarian cancer cells to produce microparticles (formation of cell vesicles encapsulating chemotherapeutic drugs)
[0045] 1. Experimental materials and reagents
[0046] H22 mouse liver cancer cells, A2780 human ovarian cancer cells, and doxorubicin (commercially available, a commonly used chemotherapy drug in clinical practice, with red fluorescence).
[0047] 2. Experimental steps
[0048] 1) Cultivate H22 mouse liver cancer cells in DMEM cell culture medium to make the cell amount reach 2×10 7 ;Cultivate A2780 human ovarian cancer cells to make the cell volume reach 2×10 7 Divide the above-mentioned H22 mouse liver cancer cell culture medium into two groups, each group including half of the amount of H22 mouse liver cancer cells; similarly divide the A2780 human ovarian cancer cell culture medium into two groups, each group including half of the amount of A2780 human o...
Embodiment 2
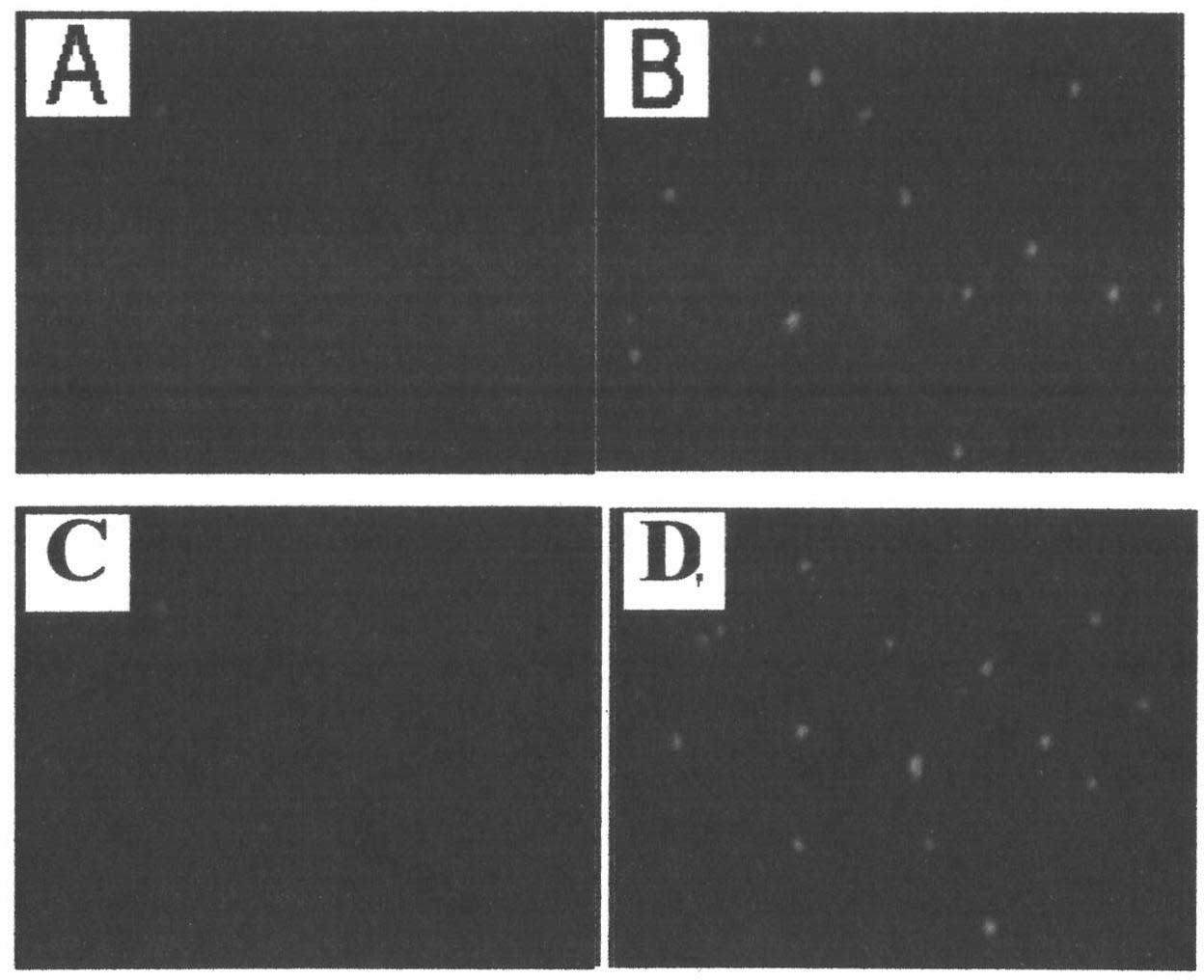
[0054] Example 2: Using ultraviolet rays to induce apoptosis of mouse liver cancer cells and human ovarian cancer cells to produce cell vesicles and incubate with chemotherapeutic drugs to obtain microparticles coated with chemotherapeutic drugs
[0055] 1. Experimental Materials and Reagents
[0056] The H22 mouse liver cancer cells and A2780 human ovarian cancer cells used were the same as in Example 1, the ultraviolet device was owned by a conventional cell clean bench, and the doxorubicin was the same as in Example 1.
[0057] 2. Experimental steps
[0058] 1) Culture H22 mouse liver cancer cells and A2780 human ovarian cancer cells to make the cell volume reach 2×10 7 , the culture method is the same as in Example 1, the above-mentioned H22 mouse liver cancer cell culture solution is divided into two groups, each group includes half of the amount of H22 mouse liver cancer cells, and the A2780 human ovarian cancer cell culture solution is also divided into two groups, eac...
Embodiment 3
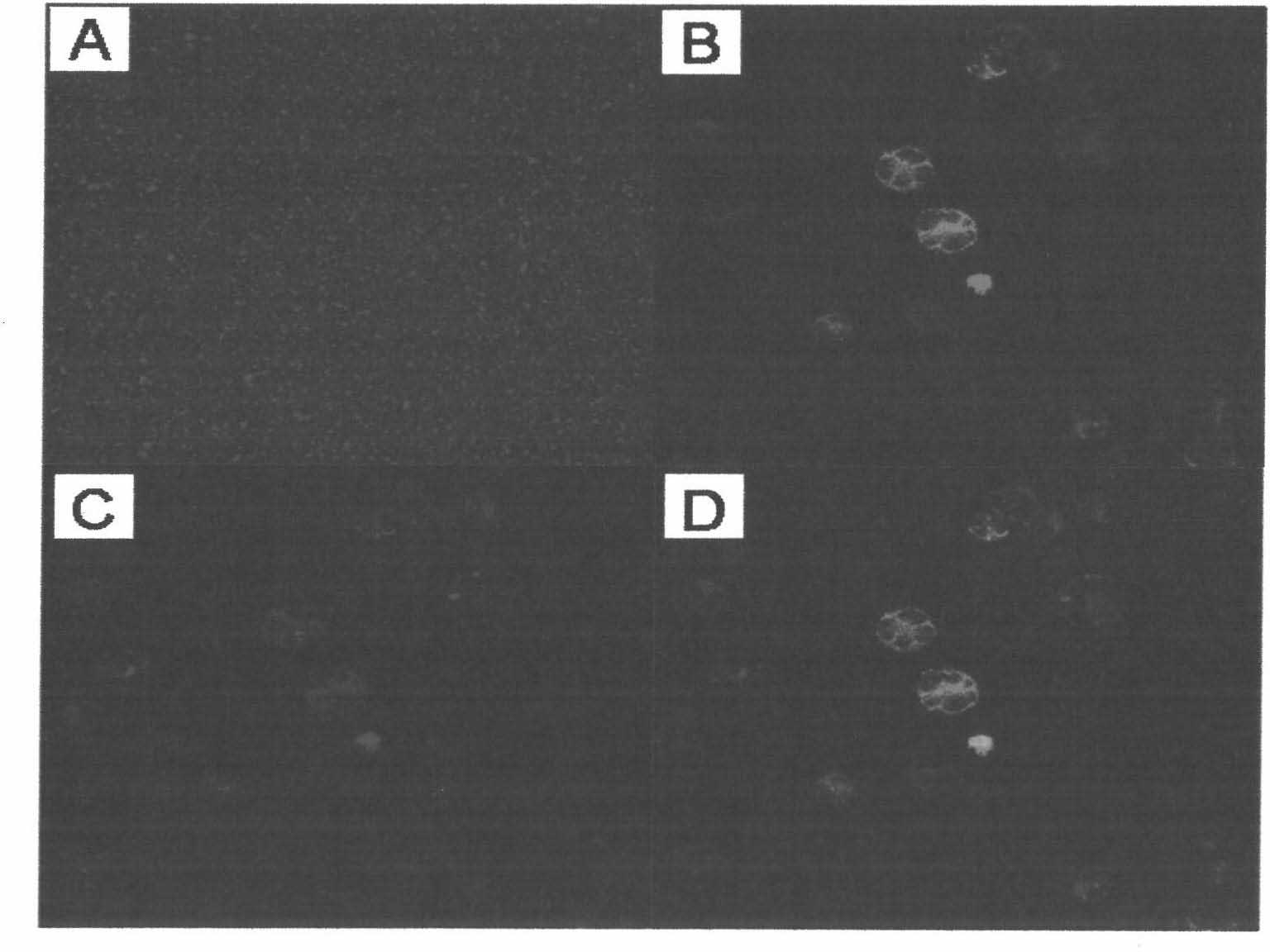
[0064] Example 3: Ultraviolet-induced cell vesicles produced by mouse liver cancer cell lines can be taken up by mouse liver cancer cells
[0065] 1. Experimental materials and reagents
[0066] The H22 mouse liver cancer cells and the ultraviolet device used were the same as in Example 1, carboxyfluorescein succinimidyl ester (CFSE) (green fluorescent dye, commercially available), PKH26 (red fluorescent dye, commercially available).
[0067] 2. Experimental steps
[0068] 1) Culture 2×10 7 Individual H22 mouse liver cancer cells were cultured in the same manner as in Example 1, and the cultured H22 mouse liver cancer cells were divided into two groups with the same amount.
[0069] 2) For the first group of cells, the green fluorescent dye CFSE was used to label according to a conventional method.
[0070] For the second group of cells, the red fluorescent dye PKH26 was used to label according to the conventional method, and the labeled H22 mouse liver cancer cells showed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com