Compounds and methods for the treatment of autoimmune and inflammatory disease
A technology for autoimmunity and diseases, applied in allergic diseases, metabolic diseases, skin diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
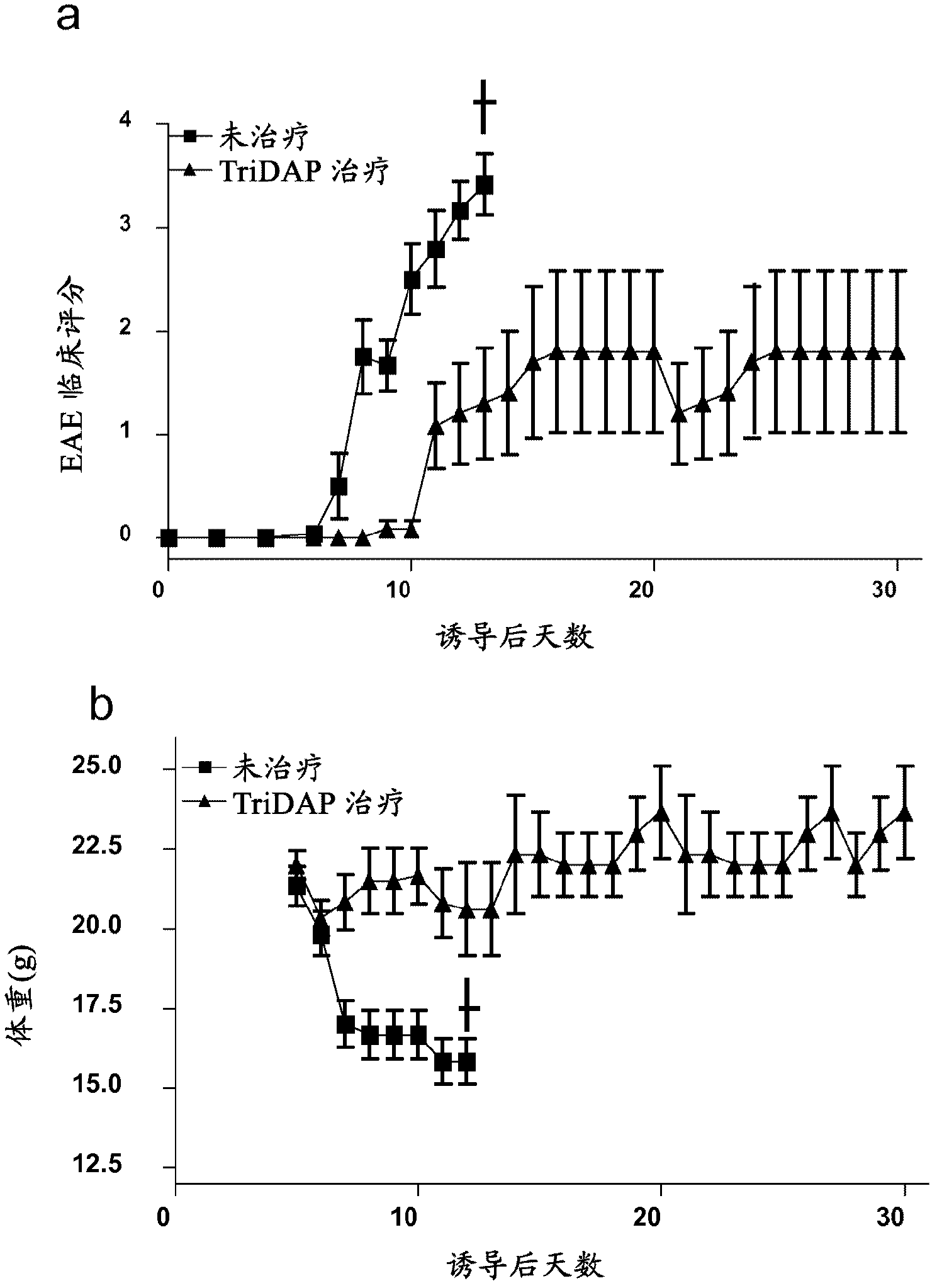
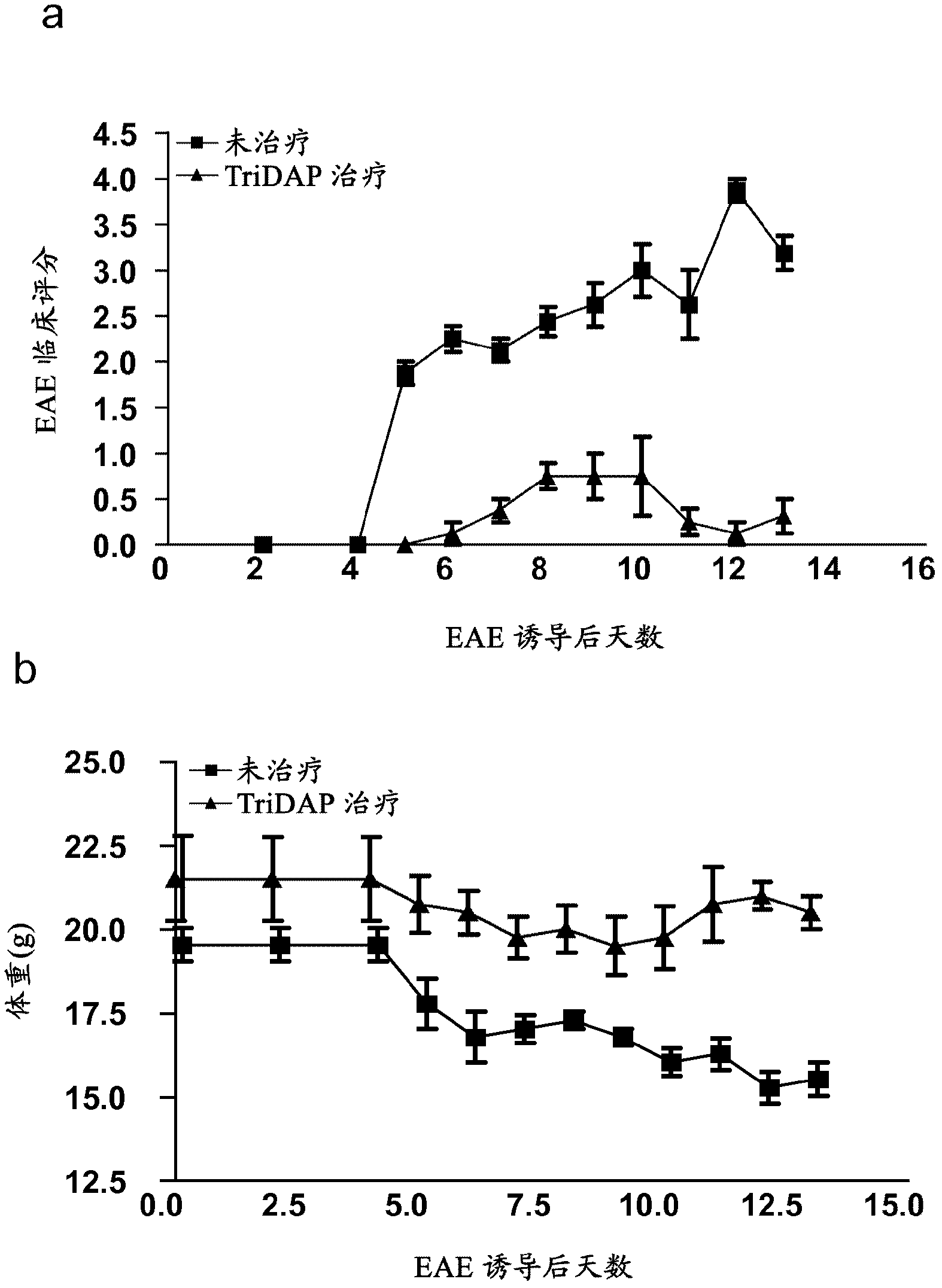
[0137] Example 1 - Treatment with the NOD1 agonist Tri-DAP attenuates experimental autoimmune cerebrospinal inflammation (EAE)
[0138] This assay was designed to identify whether Tri-DAP therapy was able to reduce clinical scores of disease severity following induction of EAE in mice.
[0139] Materials and methods:
[0140] On day 0, C57BL / 6 mice were injected subcutaneously (s.c.) with 150 μg MOG 35-55 Induced EAE, MOG 35-55 Present in CFA supplemented with 4 mg / ml H37Ra M. tuberculosis. On days 0 and 2, all mice were injected intraperitoneally (i.p.) with pertussis toxin (PT). One group of mice received no treatment and a second group was given Tri-DAP (100 μg / mouse) in MOG / CFA emulsion on day 0 and again on day 1, day 2 and every two days thereafter. Clinical scores were assessed daily and body weights were recorded. Disease severity was graded as follows: Grade 0: normal; Grade 1: tail lameness; Grade 2: gait wagging; Grade 3: hindlimb weakness; Grade 4: hindl...
Embodiment 2-E
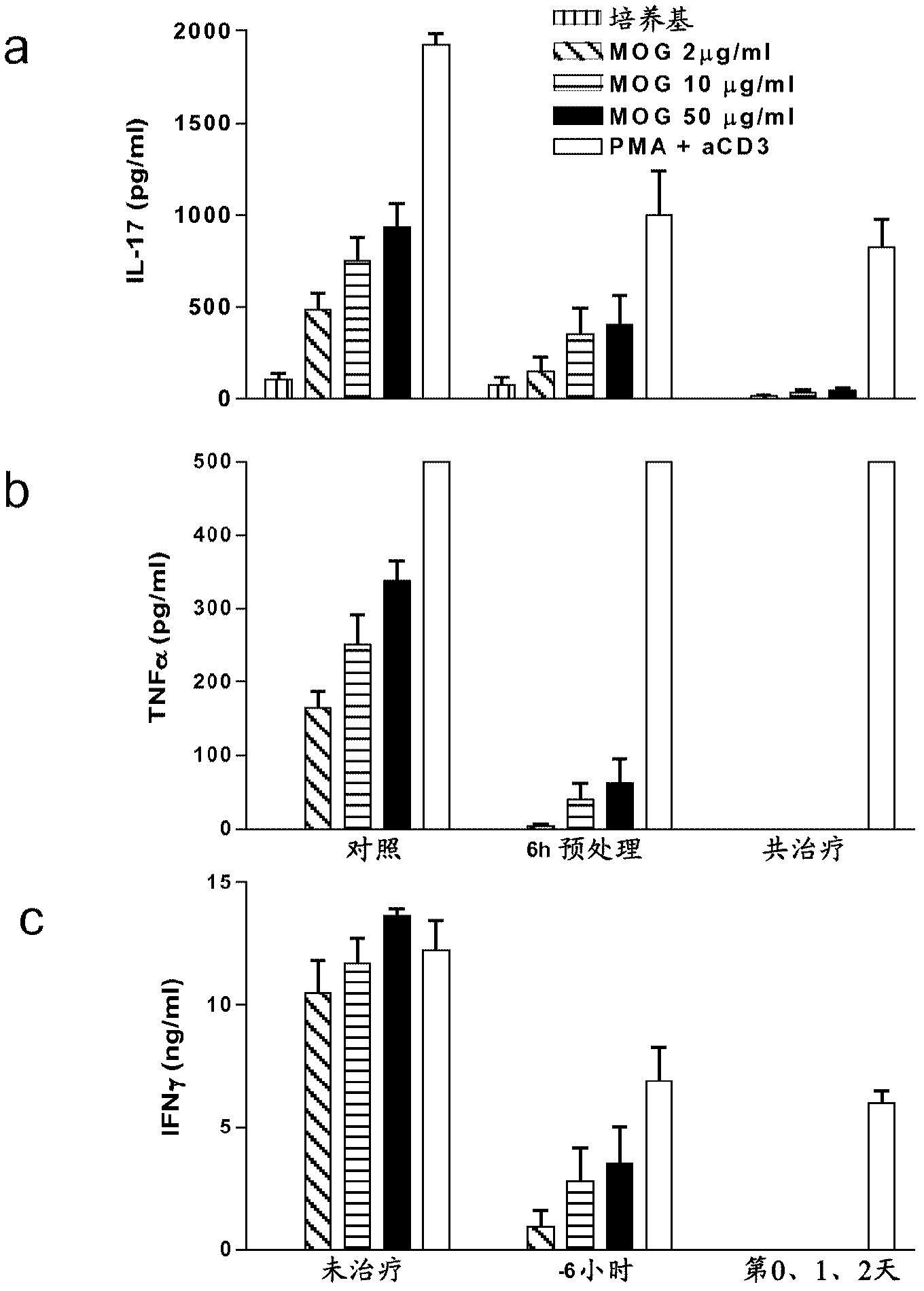
[0144] Example 2 - Treatment with NOD1 agonist Tri-DAP following EAE induction inhibits MOG specificity inflammatory cytokines
[0145] The assay was designed to determine the effect of Tri-DAP treatment on Th1 and Th17 specific inflammatory cytokine production after EAE induction.
[0146] Materials and methods:
[0147] On day 0, C57BL / 6 mice were injected subcutaneously with 150 μg MOG 35-55 Induced EAE, MOG 35-55 Present in CFA supplemented with 4 mg / ml H37Ra M. tuberculosis. On days 0 and 2, all mice were injected intraperitoneally with PT. One group of mice received no treatment, the second group was given 100 μg Tri-DAP 6 hours before immunization, the third group was given Tri-DAP (100 μg / mouse) with MOG / CFA emulsion on the 0th day, and on the 1st, 2nd day And 4 days to give again. Mice were sacrificed on day 5 and lymph node cells were stimulated with MOG peptide (myelin oligodendrocyte glycoprotein) (20-100 μg / ml) or medium alone or PMA and anti-CD3 as neg...
Embodiment 3
[0151] Example 3 - In the brain of mice with EAE, the use of NOD1 agonist Tri-DAP to treat inhibitory T cells expressing IL-17 and expressing IFNγ
[0152] The assay was designed to determine the effect of Tri-DAP treatment on various IFN-γ and IL-17 producing T cell populations in the mouse brain following EAE induction.
[0153] Materials and methods:
[0154] Twelve days after EAE induction, monocytes were isolated from the brains of control EAE or Tri-DAP-treated EAE mice. Cells were restimulated overnight with PMA / ionomycin and incubated with brefeldin A. With anti-CD3 ( Figure 4 ), or anti-CD3 and anti-CD4 ( Figure 5 and 6 ) to stain the cells. Cells were then fixed and permeabilized, stained intracellularly with anti-IFN-γ and anti-IL-17, and analyzed by flow cytometry.
[0155] result:
[0156] In all tested T cell populations ( Figure 4 CD3 + cell, Figure 5 CD3 + CD4 + cells and Figure 6 CD3 + CD4 - cells), Tri-DAP treatment inhibited the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com