Method for preparing butyraldehyde from propylene and synthesis gas
A synthesis gas and propylene technology, applied in carbon monoxide reaction preparation, chemical recovery, organic chemistry, etc., can solve the problems of low conversion rate and production capacity of the second reactor, short catalyst service life, high production cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
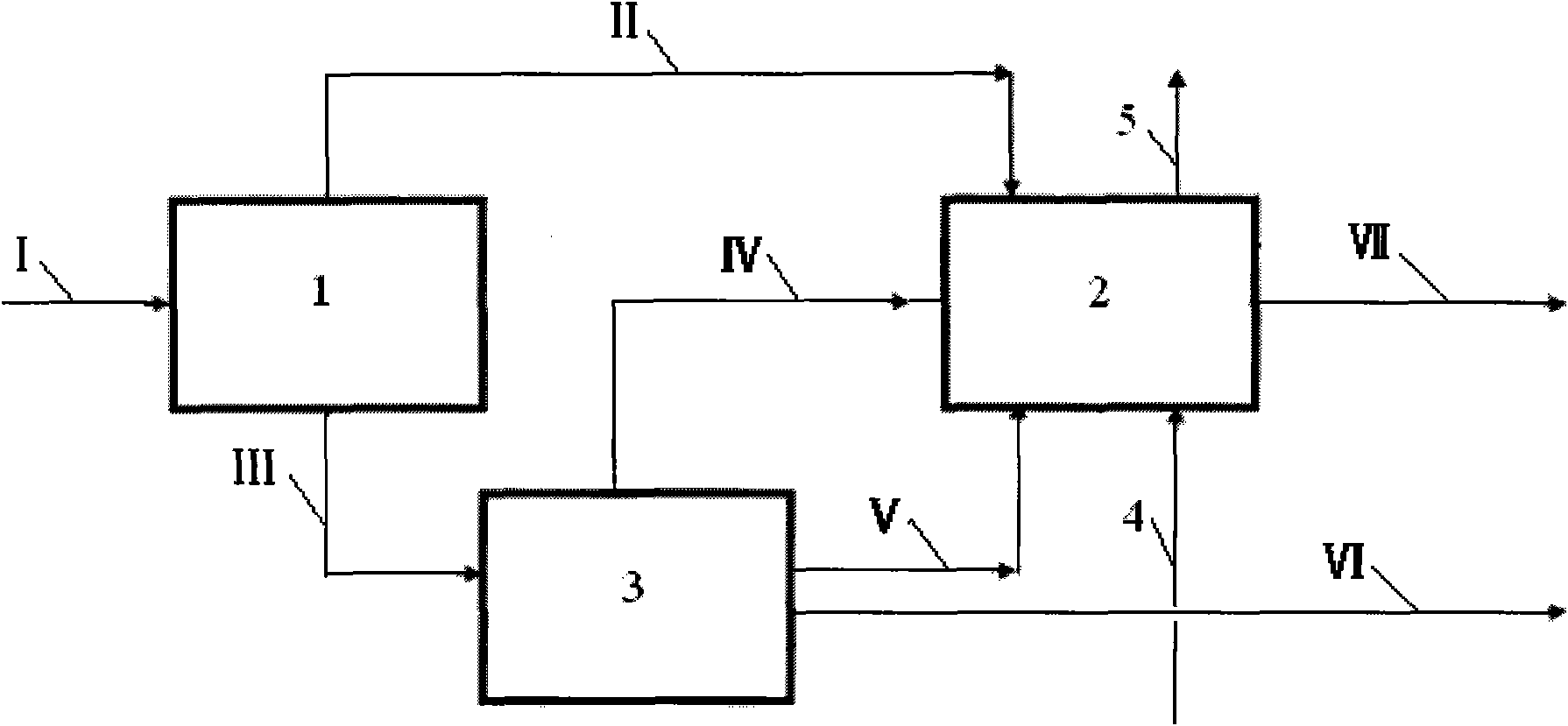
[0025] according to figure 2 In the process shown, the stream I containing propylene, synthesis gas and catalyst enters the first reactor 1. After the reaction, the vapor phase stream II is obtained at the top of the tower, and the liquid phase stream III is obtained at the bottom of the tower. Stream III enters the intermediate separation unit 3, and after separation, stream IV containing propylene and butyraldehyde, stream V containing catalyst and butyraldehyde, and crude butyraldehyde product stream VI are obtained; stream VI enters the subsequent process. Stream II, stream IV, stream V and make-up synthesis gas 4 enter the second reactor, and after the reaction, stream VII containing butyraldehyde and tail gas 5 are obtained, and tail gas 5 is discharged outside.
[0026] The operating conditions of the first reactor are: pressure 1900KPa, temperature 90°C, residence time 3 hours. The weight ratio of propylene, synthesis gas and catalyst in stream I is 1:0.57:0.29. The...
Embodiment 2
[0039] The flow process is the same as [Example 1], but the operation mode is different. Increased feed, continuous use of intermediate separation units. In this way, the production capacity can be increased and the potential of the subsequent separation device can be fully tapped without changing the structure of the reactor or the subsequent main equipment. For existing installations, the use of the present invention is very advantageous. In the case of adding a little equipment, the capacity can be expanded by up to 10%.
[0040] The reaction result is: the conversion rate of the first reactor is 65%, and the conversion rate of the second reactor is 83%. See Table 4 and Table 5 for details.
[0041] Table 4
[0042]
[0043] table 5
[0044]
[0045] Under the premise of not changing the structure, size and size of the two reactors, the production capacity of the reaction unit is increased by 10%. Nascent butyraldehyde increased from 395kmol / h to 435kmol / h. If ...
Embodiment 3
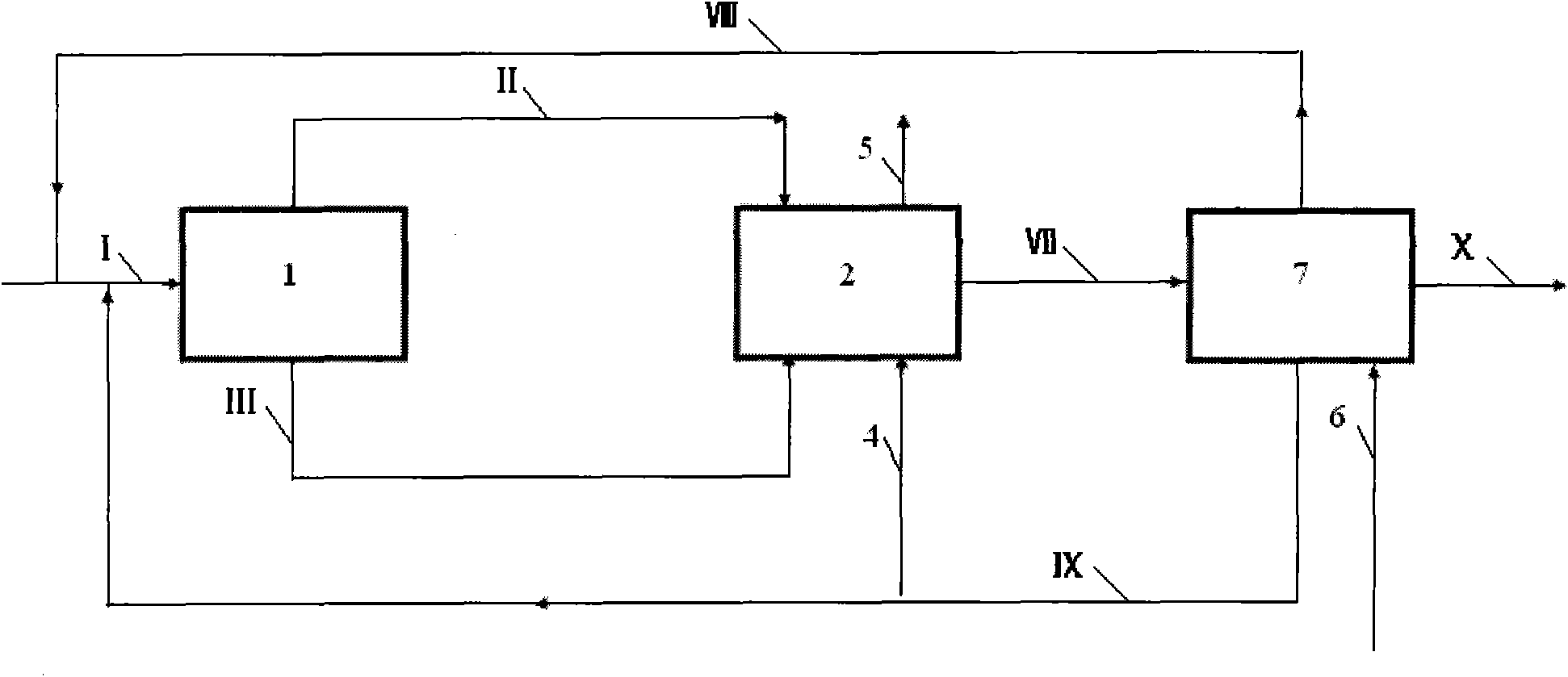
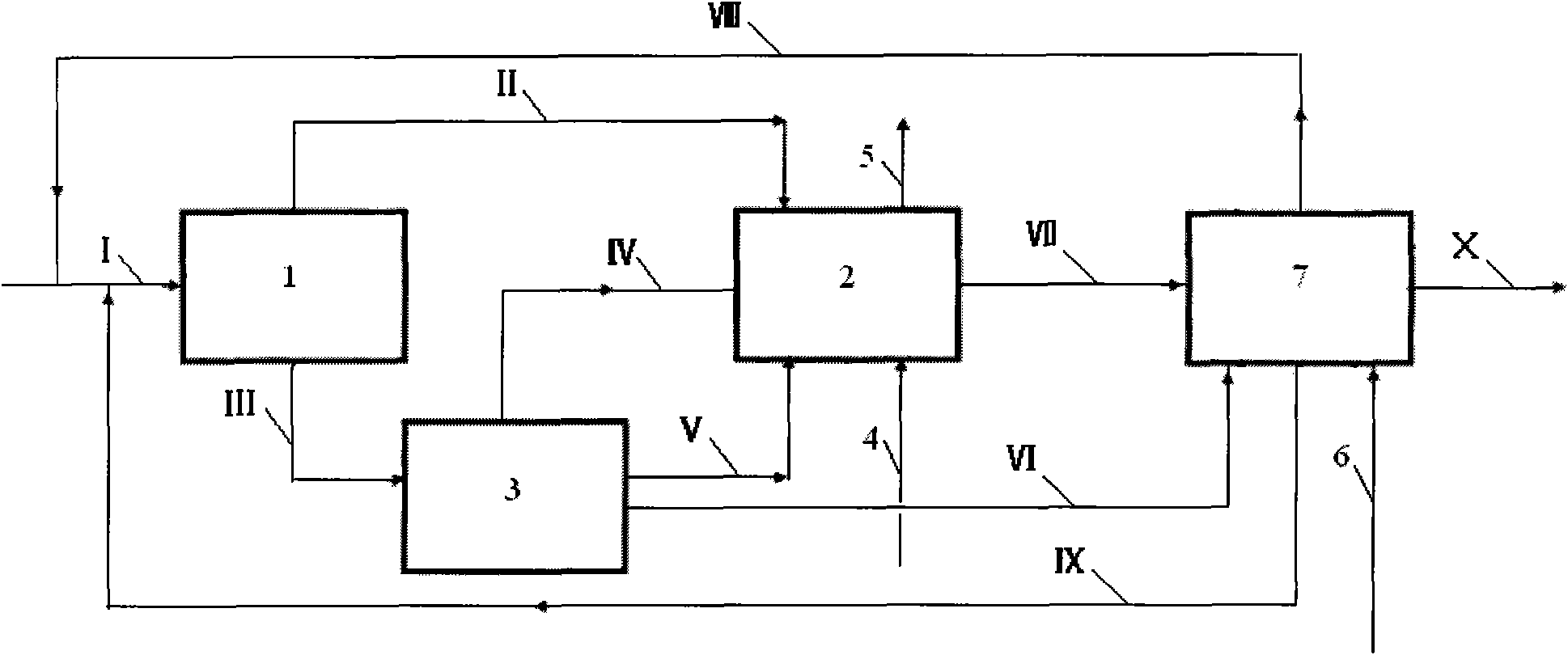
[0047] according to image 3 In the process shown, the stream I containing propylene, synthesis gas and catalyst enters the first reactor 1. After the reaction, the vapor phase stream II is obtained at the top of the tower, and the liquid phase stream III is obtained at the bottom of the tower. Stream III enters the intermediate separation unit 3, and after separation, stream IV containing propylene and butyraldehyde, stream V containing catalyst and butyraldehyde, and crude butyraldehyde product stream VI are obtained; stream VI enters the subsequent process. Stream II, stream IV, stream V and make-up synthesis gas 4 enter the second reactor, and after the reaction, stream VII containing butyraldehyde and tail gas 5 are obtained, and tail gas 5 is discharged outside. The stream VI and the stream VII enter the separation unit 7, and the synthesis gas is used as the stripping gas to obtain the vapor phase stream VIII containing propylene and the synthesis gas, the liquid phase ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com