Ureido silane compound and room temperature curable organopolysiloxane composition
A technology of ureidosilane and compound, which is applied in the field of new ureidosilane compounds, can solve the problems of reduced storage stability, catalyst deactivation, and poor curability over time, and achieves improved storage stability, excellent adhesion, and stable storage excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
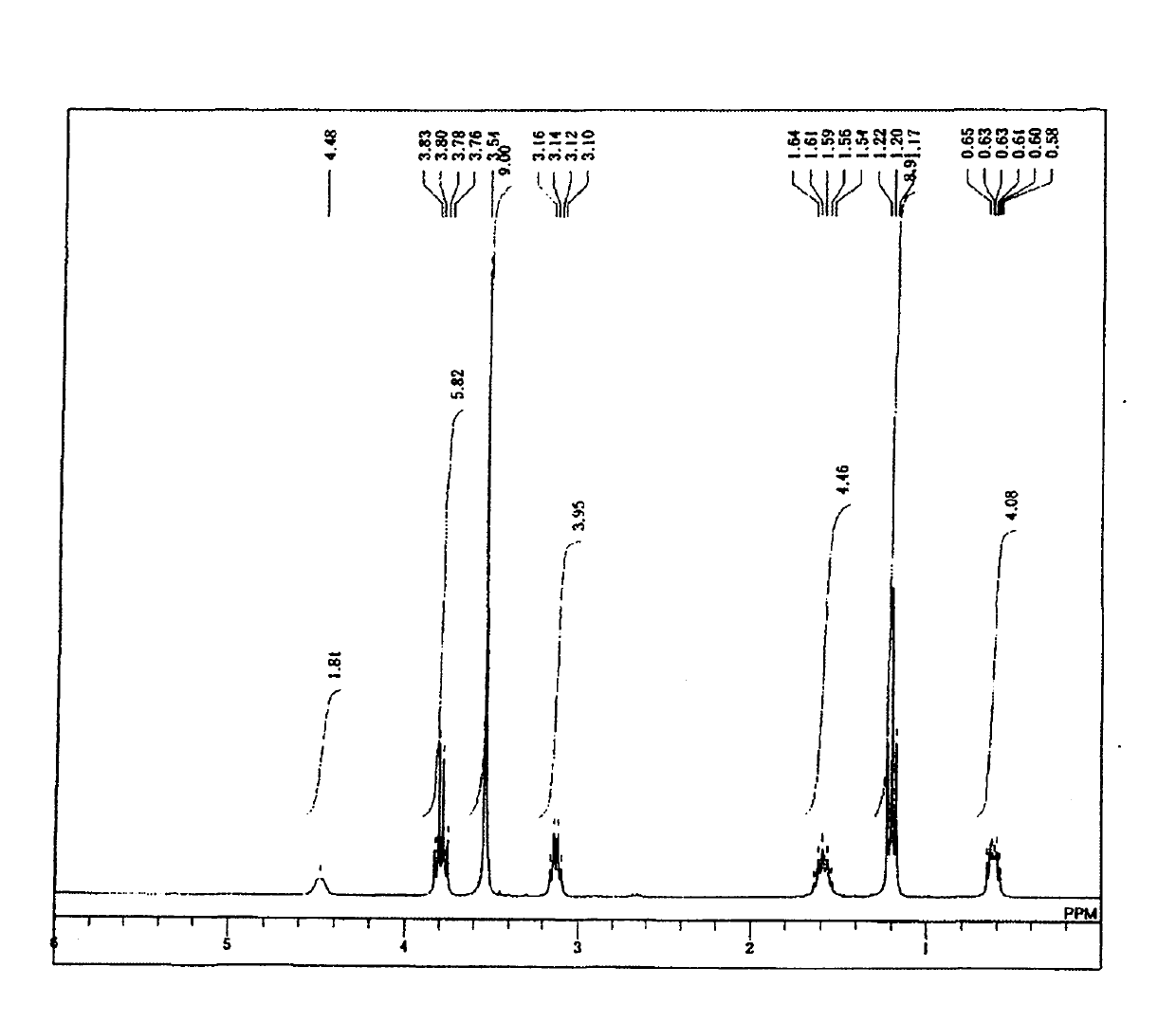
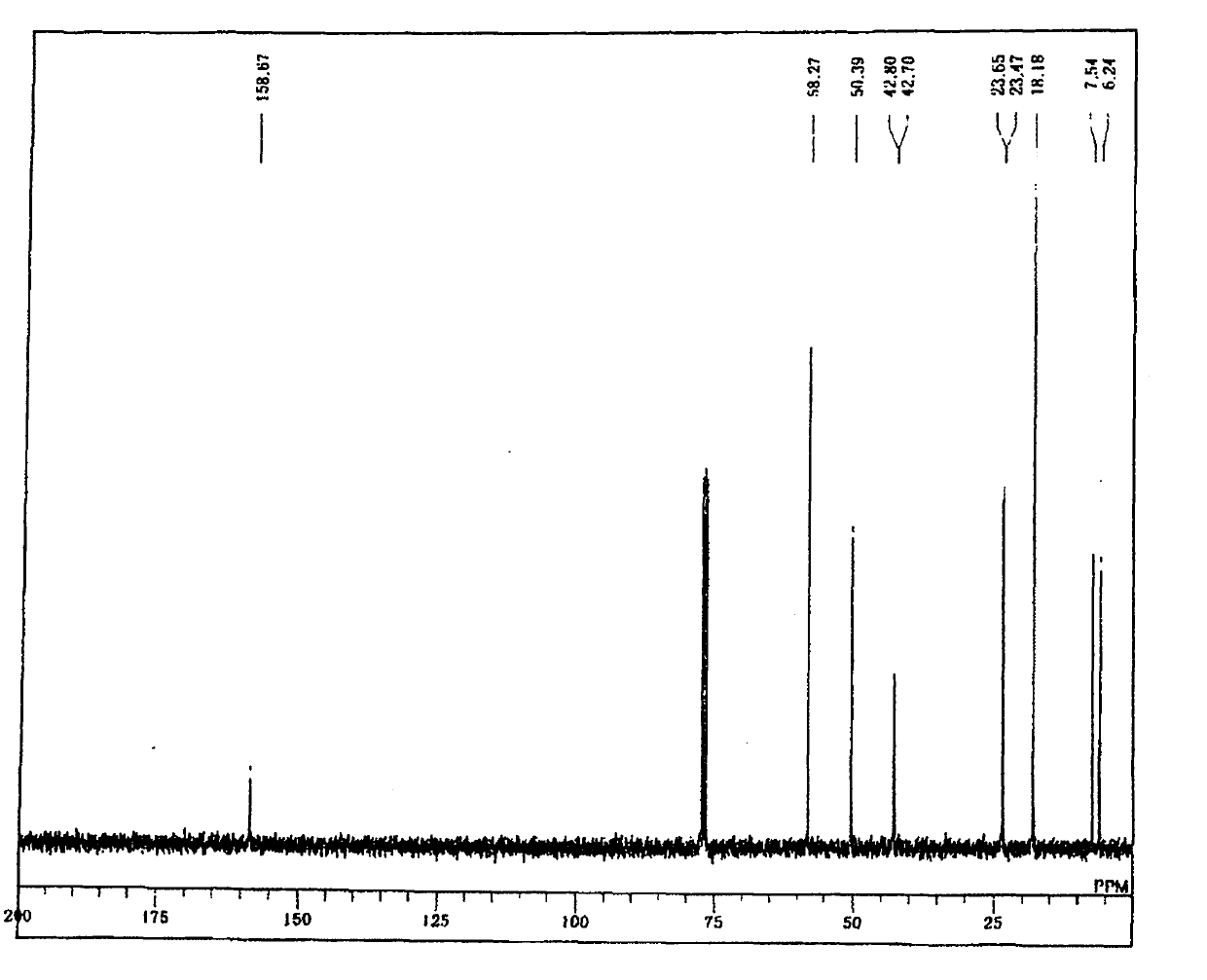
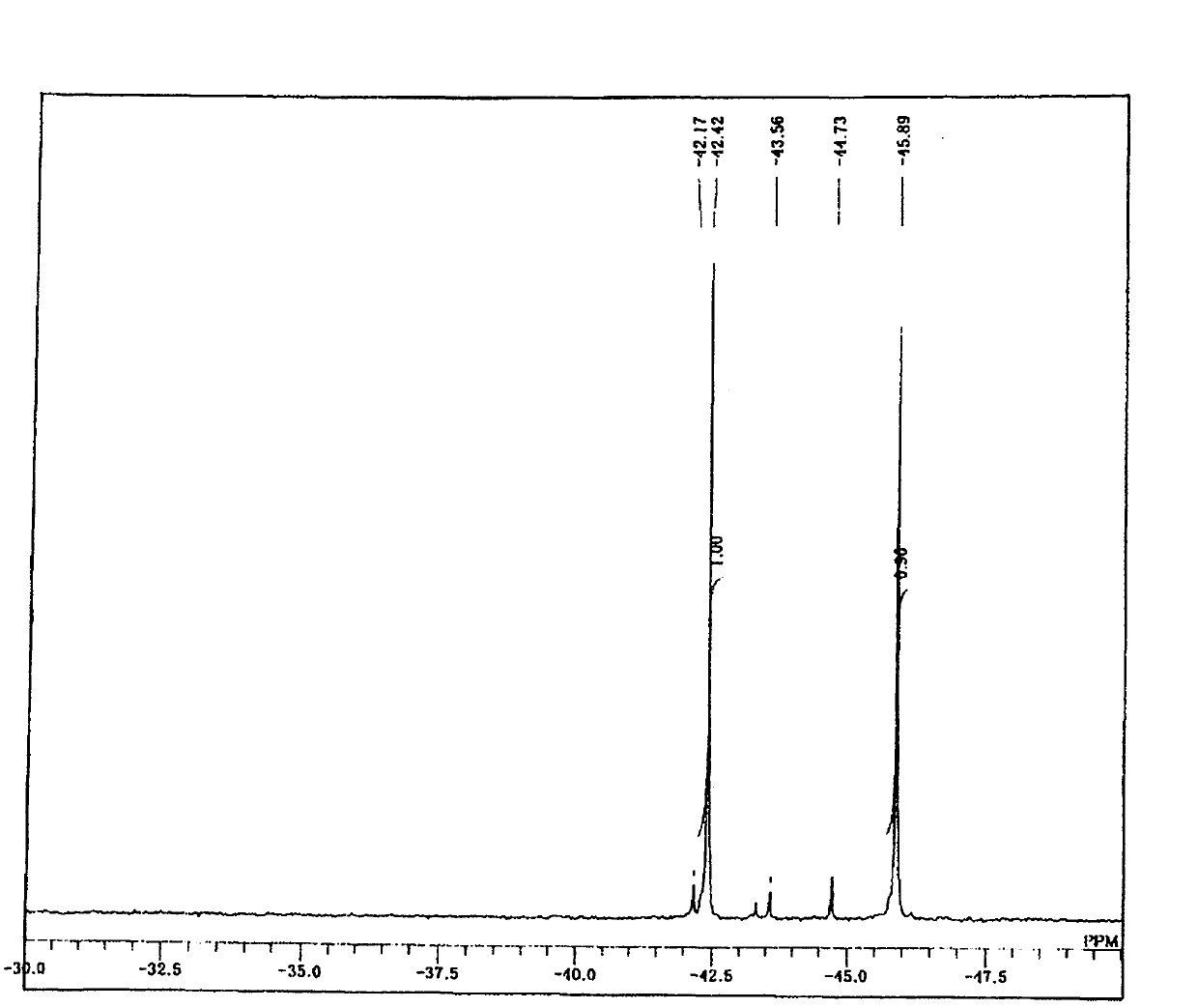
[0179] 24.7 g (0.1 mol) of 3-isocyanatopropyltriethoxysilane and 50 mL (2 M) of dichloromethane were added to a 200 mL two-necked eggplant-shaped flask equipped with a stirring element and a dropping funnel, and the mixture was kept at 0°C. In this state, 18.7 g (0.105 mol, 1.05 equivalent) of 3-aminopropyltrimethoxysilane was dripped and reacted. During the dropwise addition, heat generation was confirmed, and when the dropwise addition was terminated, the heat generation ceased, and the system was returned to room temperature. After returning to room temperature, it was further stirred for 10 minutes, dichloromethane as a solvent was distilled off, and the following target compound - formula (9) was obtained.
[0180] For the target compound-formula (9), by 1 H-NMR, 13 C-NMR, 29 Si-NMR analysis confirms that purity and yield are both 99% ( 1 H-NMR is figure 1 , 13 C-NMR is figure 2 , 29 Si-NMR is image 3 ).
[0181] 【Chemical 25】
[0182]
Embodiment 2
[0184] 24.7 g (0.1 mol) of 3-isocyanatopropyltriethoxysilane and 50 mL (2 M) of dichloromethane were added to a 200 mL two-necked eggplant-shaped flask equipped with a stirring element and a dropping funnel, and the mixture was kept at 0°C. In this state, 6.8 g (0.05 mol, 0.5 equivalent) of m-xylylenediamine was dropped and reacted. During the dropwise addition, heat generation was confirmed, and when the dropwise addition was terminated, the heat generation ceased, and the system was returned to room temperature. After returning to room temperature, it was further stirred for 10 minutes, dichloromethane as a solvent was distilled off, and the following target compound - formula (10) was obtained.
[0185] For the target compound-formula (10), by 1 H-NMR, 13 C-NMR, 29 Si-NMR analysis confirms that purity and yield are both 99% ( 1 H-NMR is Figure 4 , 13 C-NMR is Figure 5 , 29 Si-NMR is Figure 6 ).
[0186] 【Chemical 26】
[0187]
Embodiment 3
[0189] 24.7 g (0.1 mol) of 3-isocyanatopropyltriethoxysilane and 50 mL (2 M) of dichloromethane were added to a 200 mL two-necked eggplant-shaped flask equipped with a stirring element and a dropping funnel, and the mixture was kept at 0°C. In this state, bis[3-trimethoxysilylpropyl]amine (KBM-666P, manufactured by Shin-Etsu Chemical Co., Ltd.) was dropped and reacted. During the dropwise addition, heat generation was confirmed, and when the dropwise addition was terminated, the heat generation ceased, and the system was returned to room temperature. After returning to room temperature, it was further stirred for 10 minutes, dichloromethane as a solvent was distilled off, and the following target compound - formula (11) was obtained.
[0190] With regard to the target compound-formula (11), by 1 H-NMR, 13 C-NMR, 29 Si-NMR analysis confirms that purity and yield are both 99% ( 1 H-NMR is Figure 7 , 13 C-NMR is Figure 8 , 29 Si-NMR is Figure 9 ).
[0191] 【Chemical ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com