Injectable drug combination and preparation method thereof
A composition and injection technology, which can be used in drug combinations, antipyretics, antitumor drugs, etc., can solve problems such as affecting cardiovascular function, long-term safety inspection of stabilizers and excipients, and reduce potential safety hazards. , good safety, the effect of preventing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of freeze-dried powder injection:
[0032] 29.5g Na 3 PO 4 Dissolve in 1800ml water for injection to make 0.1mol / L Na 3 PO 4 solution; another appropriate amount of 1mol / L NaOH solution was prepared for subsequent use; 200g salvianolic acid B was weighed, and the above-mentioned 0.2mol / L NaOH solution was added 3 PO 4 Solution 1800ml, after stirring evenly, then add dropwise the above 1mol / L NaOH solution to pH 4.0, add 0.5% activated carbon for injection to remove heat source, add water for injection to 2000ml. Filter with a 0.22um microporous membrane, dispense into 10ml freeze-drying bottles, and freeze-dry.
[0033] Freeze-drying curve:
[0034] -40°C, 5h (minimum eutectic point -12.3°C)
[0035] -25℃, 14h
[0036] 0°C, 3h
[0037] 20℃, 2h
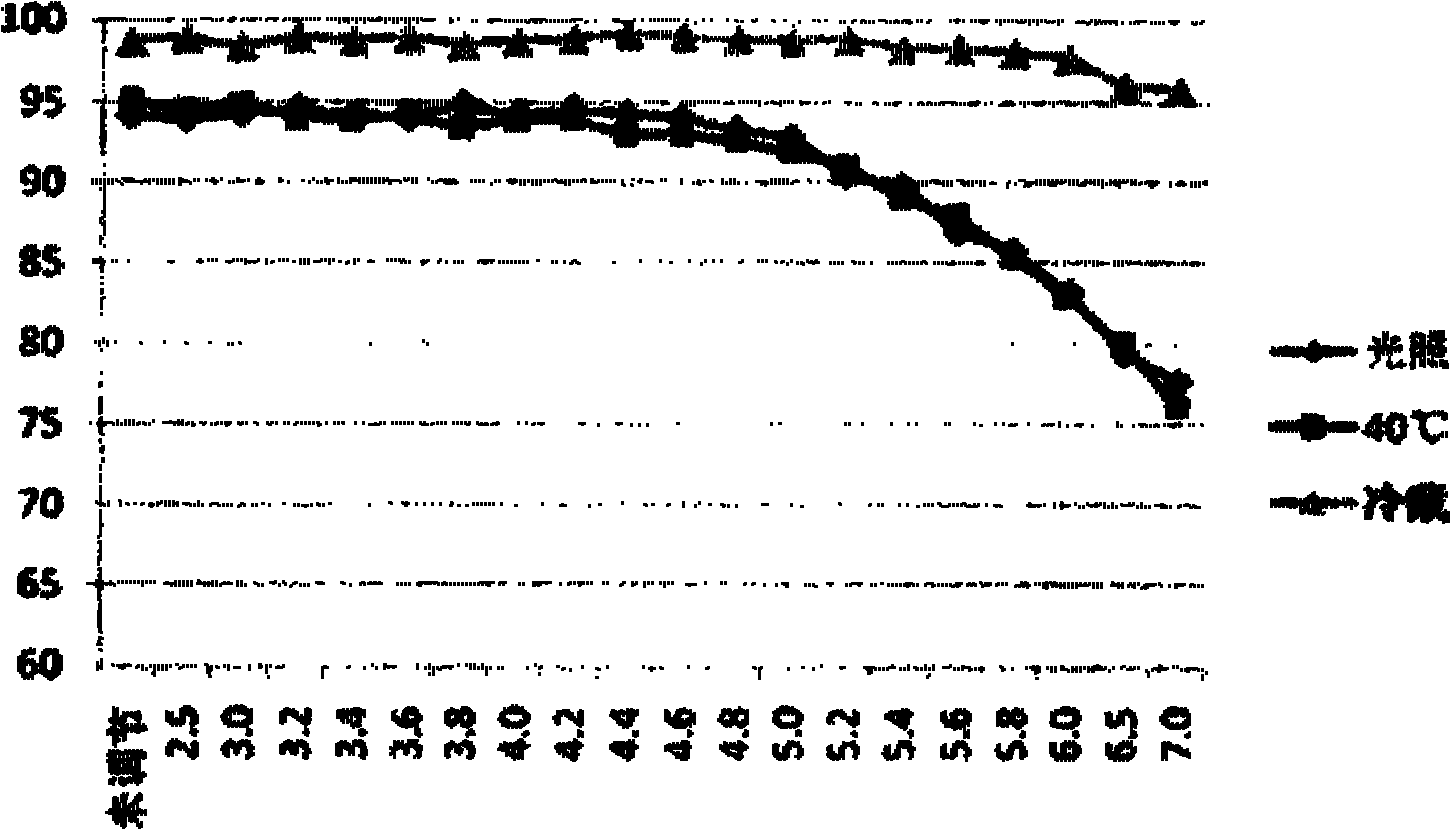
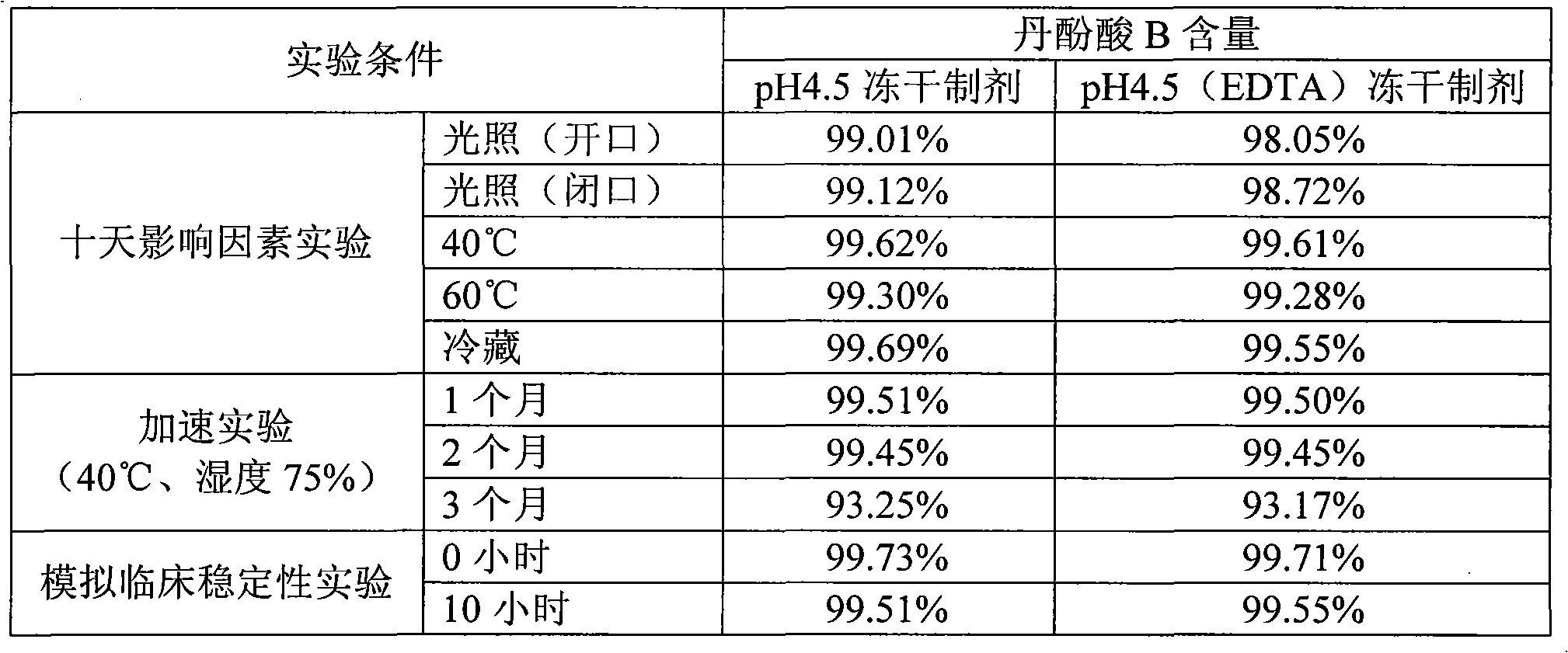
[0038] Stability: The freeze-dried powder injection of salvianolic acid B was placed under the accelerated conditions of temperature 40°C and relative humidity 75% for three months. Taking the crude drug a...
Embodiment 2
[0040] Dissolve 14.4g NaOH in 1800ml water for injection to make a 0.2mol / L NaOH solution; prepare an appropriate amount of 1mol / L NaOH solution for later use; weigh 200g salvianolic acid B and add the above 0.2mol / L NaOH solution 1800ml, after stirring evenly, then dropwise add the above 1mol / L NaOH solution to pH 5.0, add 0.5% activated carbon for injection to remove heat source, add water for injection to 2000ml. Filter with a 0.22um microporous membrane, dispense into 10ml freeze-drying bottles, and freeze-dry.
[0041] Freeze-drying curve:
[0042] -40°C, 5h (minimum eutectic point -8.3°C)
[0043] -20℃, 14h
[0044] 0°C, 3h
[0045] 20℃, 2h
[0046] Stability: The freeze-dried powder injection of salvianolic acid B was placed under the accelerated conditions of temperature 40°C and relative humidity 75% for three months. Taking the crude drug as the standard, the content of salvianolic acid B in the sample was determined by the HPLC external standard method, and it ...
Embodiment 3
[0048] Dissolve 14.4g NaOH in 1800ml water for injection to make a 0.2mol / L NaOH solution; prepare an appropriate amount of 1mol / L NaOH solution for later use; weigh 200g salvianolic acid B and add the above 0.2mol / L NaOH solution 1800ml, after stirring evenly, then dropwise add the above 1mol / L NaOH solution to pH 7.0, add 0.5% activated carbon for injection to remove heat source, add water for injection to 2000ml. Filter with a 0.22um microporous membrane, dispense into 10ml freeze-drying bottles, and freeze-dry.
[0049] Freeze-drying curve:
[0050] -40°C, 5h (minimum eutectic point -8.3°C)
[0051] -20℃, 14h
[0052] 0°C, 3h
[0053] 20℃, 2h
[0054]Stability: The freeze-dried powder injection of salvianolic acid B was placed under the accelerated conditions of temperature 40°C and relative humidity 75% for three months. Taking the crude drug as the standard, the content of salvianolic acid B in the sample was determined by the HPLC external standard method, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com