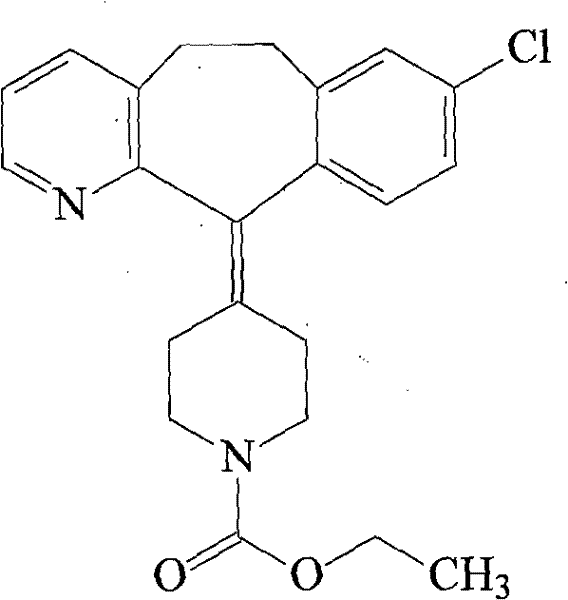
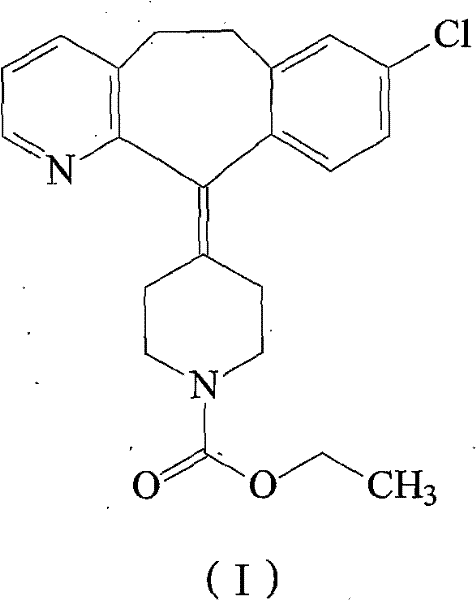
Loratadine compound and preparation method thereof
A technology of loratadine and compounds, applied in the field of loratadine compounds and its purification process, can solve the problems of reduced yield of purified products or intermediates, low purity of target products, less purity can be obtained, etc. Achieve the effects of easy control and industrialized production, low cost, and reduced toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Dissolve 10g of crude loratadine with a purity of 94.8% in 100ml of isopropanol, then add 0.1g of activated carbon, heat and stir at 30°C for 10 minutes, and filter to obtain primary purified loratadine; After the solution is concentrated, add 10g of pretreated DA-201 type macroporous adsorption tree, stir and mix well, add to the top of DA-201 type macroporous adsorption resin column, first wash with 1-2 column volumes of purified water until it is clear , And then eluted with 50% isopropanol containing 0.01mol / L hydrochloric acid, collected the eluate, concentrated under reduced pressure to about two-fifths of the volume to obtain secondary purified loratadine; stirred at 50-60°C Next, add ammonia water to the concentrated solution to pH 8.0, cool to 0~4°C overnight, separate out crystals, centrifuge, wash, and dry to obtain 9.07 g of three-stage purified loratadine with a purity of 99.63% and a yield of 95.70 %. MP: 135~136℃
Embodiment 2
[0043] Dissolve 10g of crude loratadine with a purity of 94.8% in 100ml of isopropanol, then add 0.3g of activated carbon, heat and stir at 40°C for 15 minutes, and filter to obtain primary purified loratadine; After the solution is concentrated, add 10g of the pretreated Diaion HP2MG type macroporous adsorption tree, stir and mix well, add to the top of the Diaion HP2MG type macroporous adsorption resin column, first wash with 1 to 2 column volumes of purified water until it is clear, then Elute with 50% isopropanol containing 0.01mol / L hydrochloric acid, collect the eluate, concentrate under reduced pressure to about two-fifths of the volume to obtain secondary purified loratadine; Ammonia water was added to the concentrated solution to pH 8.2, cooled to 0-4°C overnight, crystals were precipitated, centrifuged, washed, and dried to obtain 9.03 g of three-stage purified loratadine with a purity of 99.75% and a yield of 95.28%. MP: 135~136℃.
Embodiment 3
[0045] Dissolve 10g of crude loratadine with a purity of 94.8% in 100ml of isopropanol, then add 0.4g of activated carbon, heat and stir at 50°C for 20 minutes, and filter to obtain primary purified loratadine; After the solution is concentrated, add 10g of pretreated AB-8 type macroporous adsorption tree, stir and mix well, add to the top of the AB-8 type macroporous adsorption resin column, first wash with 1 to 2 column volumes of purified water until it is clear , And then eluted with 50% isopropanol containing 0.01mol / L hydrochloric acid, collected the eluate, concentrated under reduced pressure to about two-fifths of the volume to obtain secondary purified loratadine; stirred at 50-60°C Add ammonia water to the concentrated solution until the pH value is 8.5, cool to 0~4℃ overnight, precipitate crystals, centrifuge, wash, and dry to obtain 8.99 g of three-stage purified loratadine with a purity of 99.87% and a yield of 94.8 %. MP: 135~136℃.
[0046] The following lists som...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com