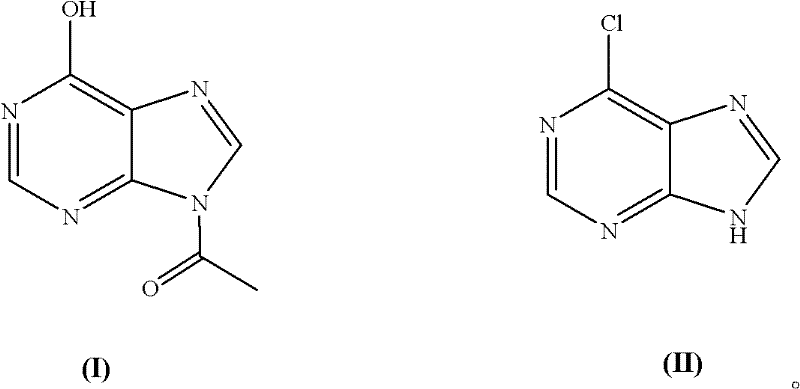
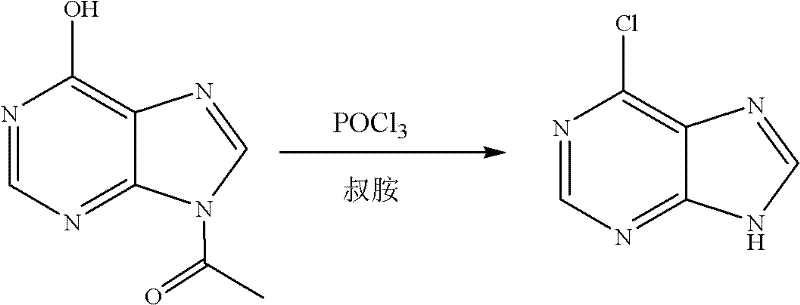
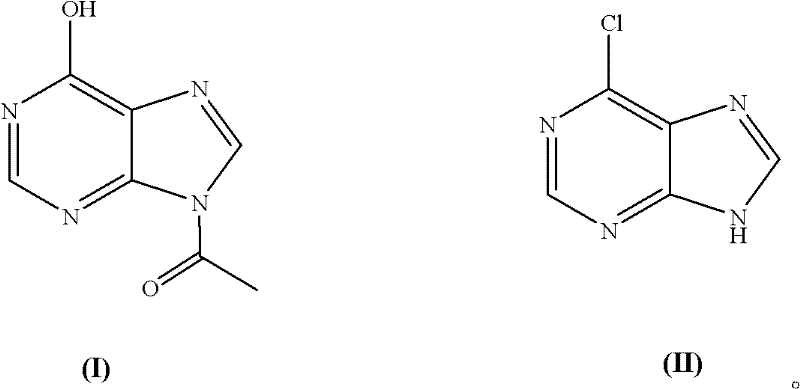
Chemical synthesis method of 6-chloropurine
A technology for chemical synthesis and chloropurine, applied in the field of chemical synthesis of 6-chloropurine, can solve problems such as environmental pollution, and achieve the effects of reducing a large amount of waste water, high yield, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: The molar ratio of feeding materials is acetyl hypoxanthine: phosphorus oxychloride: N, N-dimethylaniline is 1.0: 5.0: 1.0
[0020] In a 500mL three-necked flask equipped with a thermometer and mechanical stirring, add 35.6g acetyl hypoxanthine (0.20mol), 152153.0g phosphorus oxychloride (1.00mol), 24.2g N,N-dimethylaniline (0.20 mol), start stirring, heat to 105°C, keep warm for 4 hours, and evaporate unreacted phosphorus oxychloride. Cool to 0°C, add 100 mL of ice water, and adjust the pH to 8 with 0.1M NaOH solution. After cooling, a large amount of yellow solid precipitated out. Filter, wash with cold water, and dry to obtain 27.6 g of 6-chloropurine, with a yield of 90.0% (calculated as acetyl hypoxanthine, the same below.), and a purity of 99.0% after testing (high performance liquid chromatography area normalization method, referred to as HPLC, the same below). MS (EI): M (100), M+2 (33).
Embodiment 2
[0021] Example 2 The amount ratio of the feeding material is changed to acetyl hypoxanthine: phosphorus oxychloride: N, N-dimethylaniline is 1.0: 20.0: 1.0
[0022] In a 500mL three-neck flask equipped with a thermometer and mechanical stirring, add 35.6g acetyl hypoxanthine (0.20mol), 612.0g phosphorus oxychloride (4.00mol), 24.2g N,N-dimethylaniline (0.20 mol), start stirring, heat to 105°C, keep warm for 4 hours, and evaporate unreacted phosphorus oxychloride. Cool to 0°C, add 100 mL of ice water, and adjust the pH to 8 with 0.1M NaOH solution. After cooling, a large amount of yellow solid precipitated out. After filtering, washing and drying, 27.3 g of 6-chloropurine was obtained, with a yield of 89.2% and a purity of 99.0% after testing.
Embodiment 3
[0024] The amount ratio of the feed material is changed to acetyl hypoxanthine: phosphorus oxychloride: N, N-dimethylaniline is 1.0: 10.0: 1.0, in a 500mL three-necked flask equipped with a thermometer and mechanical stirring, add 35.6g Acetyl hypoxanthine (0.20mol), 306.0g phosphorus oxychloride (2.00mol), 24.2g N,N-dimethylaniline (0.20mol), other conditions and preparation steps are the same as in Example 1. 27.9 g of 6-chloropurine was obtained with a yield of 91.2% and a purity of 99.0% after testing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com