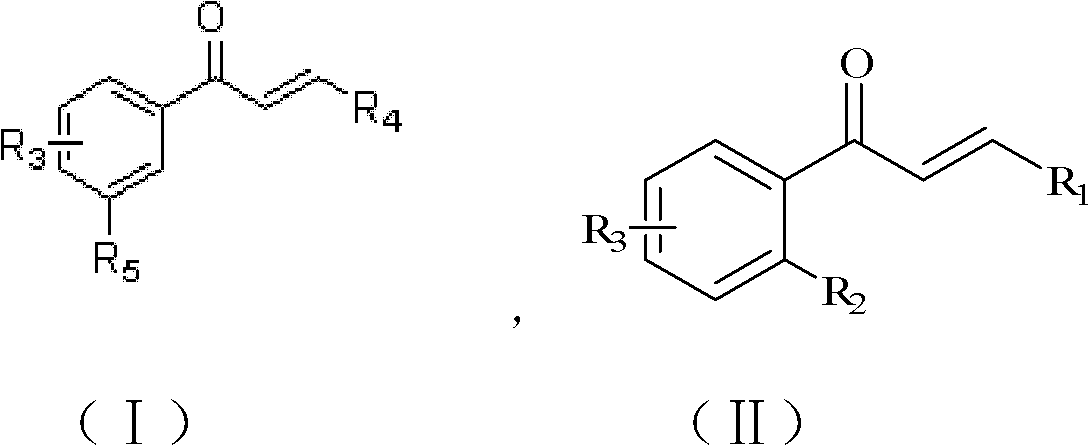
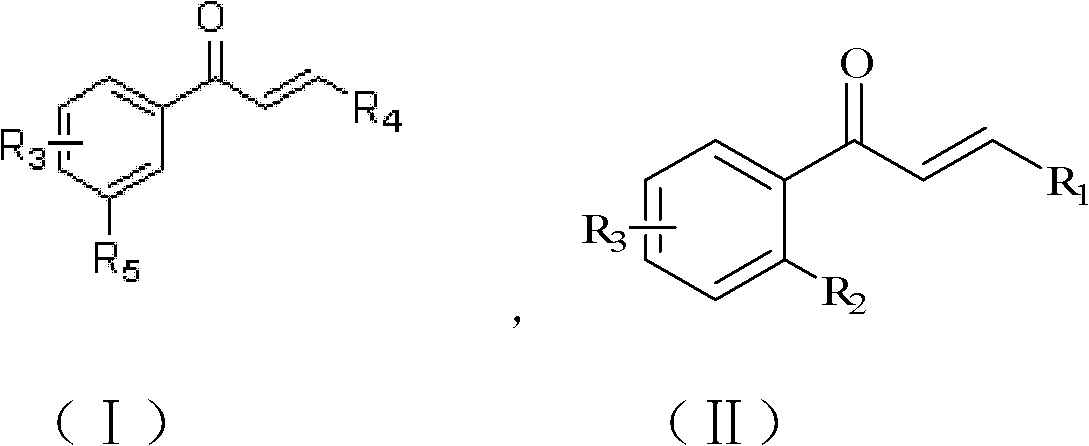
Aryl-substituted chalcones compound, its preparation method and its application
A technology of chalcones and compounds, applied in the field of aryl-substituted chalcones and their preparation, can solve the problem of low anti-tumor activity of chalcones and achieve high inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
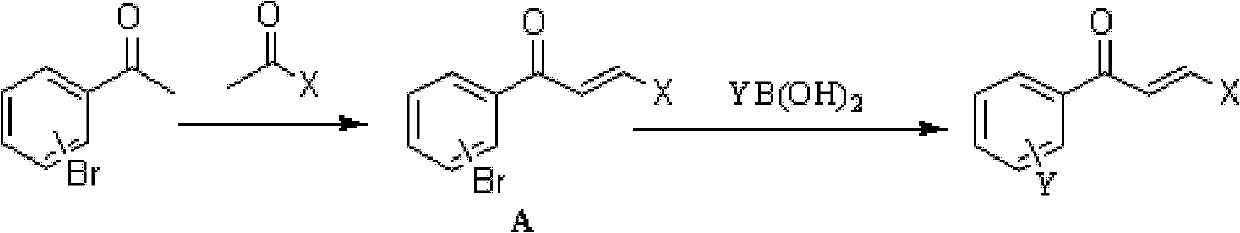
[0039] Example 1 (E)-3-(4-methoxyphenyl)-1-(3'-fluoro-[1,1'-biphenyl]-3-yl)prop-2-ene-1- Ketone (compound 1) synthesis:
[0040] 4eq m-bromoacetophenone and 5.2eq of 3-fluorophenylboronic acid with dioxane and 2M K 2 CO 3 Aqueous 1:1 mixed solution as solvent, add 0.2eq PdCl 2 (dppf), microwave heating at 150°C for 15 minutes, extraction of the organic layer with ethyl acetate, separation by column chromatography to obtain the intermediate 1-(3'-fluoro-[1,1'-biphenyl]-3-yl)ethanone , 1eq of the intermediate and 1eq of 4-methoxybenzaldehyde were dissolved in ethanol, 3eq of KOH was added as a catalyst, stirred at room temperature for 20h, adjusted to pH 3 with 1N HCl, extracted with ethyl acetate, and the organic layer was separated by column chromatography to obtain the product (E)-3-(4-methoxyphenyl)-1-(3′-fluoro-[1,1′-biphenyl]-3-yl)prop-2-en-1-one, producing rate of 65%.
[0041] Product NMR analysis:
[0042] 1 H NMR (500MHz, CDCl 3)δ8.19 (t, J=1.7Hz, 1H), 8.00 (dd...
Embodiment 2
[0043] Example 2 (E)-3-(4-methoxyphenyl)-1-(4'-fluoro-[1,1'-biphenyl]-2-yl)prop-2-ene-1- Ketone (compound 3) synthesis:
[0044] Dissolve 4eq of o-bromoacetophenone and 4eq of 4-methoxybenzaldehyde in ethanol, add 12eq of KOH as a catalyst, stir at room temperature for 20h, adjust the pH to 3 with 1N HCl, extract with ethyl acetate, and separate the organic layer by column chromatography Obtained intermediate (E)-3-(4-methoxyphenyl)-1-(2-bromophenyl)prop-2-en-1-one, 1 eq of intermediate and 1.3 eq of 4-fluorophenylboronic acid with dioxane and 2M K 2 CO 3 Aqueous solution 1:1 mixed solution as solvent, add 0.05eq PdCl 2 (dppf), microwave heating at 150°C for 15 minutes, extraction of the organic layer with ethyl acetate, separation by column chromatography to obtain the product (E)-3-(4-methoxyphenyl)-1-(4'-fluoro-[1 , 1'-biphenyl]-2-yl)prop-2-en-1-one, yield 76%.
[0045] Product NMR analysis:
[0046] 1 H NMR (500MHz, CDCl 3 )δ7.61(dd, J=7.6, 1.4Hz, 1H), 7.54(td, J=7...
Embodiment 3
[0047] Example 3 (E)-3-(4-methoxyphenyl)-1-(3'-fluoro-[1,1'-biphenyl]-2-yl)prop-2-ene-1- Ketone (compound 11) synthesis:
[0048] 4.8eq o-bromophenylboronic acid and 4eq of 3-bromofluorobenzene with dioxane and 2M K 2 CO 3 Aqueous 1:1 mixed solution as solvent, add 0.2eq PdCl 2 (dppf), microwave heating at 150°C for 15 minutes, extraction of the organic layer with ethyl acetate, separation by column chromatography to obtain the intermediate 1-(3'-fluoro-[1,1'-biphenyl]-2-yl)ethanone , 1eq of the intermediate and 1eq of 4-methoxybenzaldehyde were dissolved in ethanol, 3eq of KOH was added as a catalyst, stirred at room temperature for 20h, adjusted to pH 3 with 1N HCl, extracted with ethyl acetate, and the organic layer was separated by column chromatography to obtain the product (E)-3-(4-methoxyphenyl)-1-(3′-fluoro-[1,1′-biphenyl]-2-yl)prop-2-en-1-one, producing rate of 72%.
[0049] Product NMR analysis:
[0050] 1 H NMR (400MHz, CDCl 3 )δ8.17(t, J=1.7Hz, 1H), 8.01-7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com