Pharmaceutical composition comprising racemic aminopterin
A technology of aminopterin and composition, applied in the field of pharmaceutical compositions comprising racemic aminopterin, can solve the problem of high cost, achieve the effects of low production cost, increase system exposure, and reduce regulatory burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] pharmaceutical composition
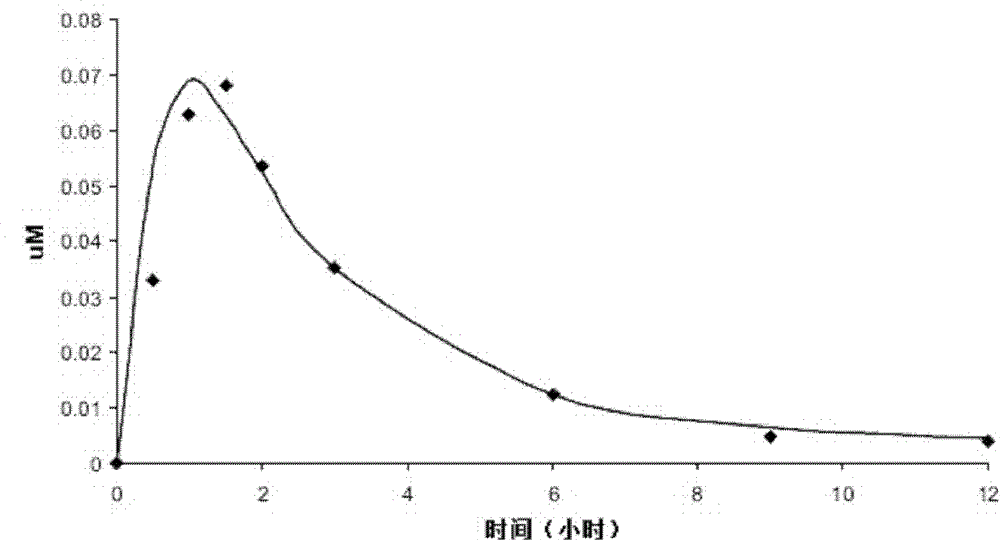
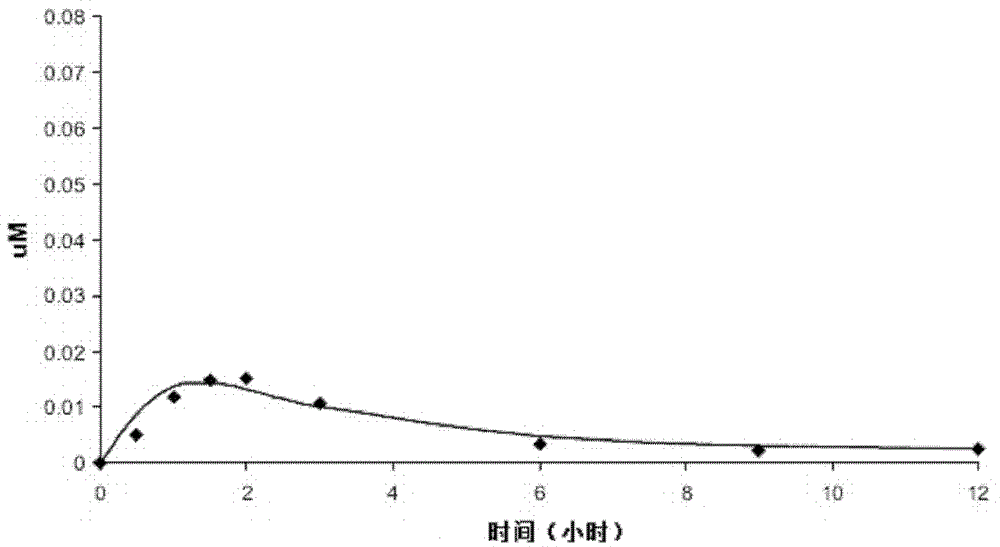
[0063] See Table 1 for optically pure, racemic, scored, immediate release (IR) tablet formulations in dosage strengths of 0.25 mg (Lot No. 157I0907) and 1.0 mg (Lot Nos. 387I1100 and 116I0604).
[0064] Table 1
[0065]
[0066] * In any event, the drug in the tablet is the disodium salt and the dosage strength required for the tablet is the free acid (ie diacid) equivalents of the L-isomer or the D-isomer plus the L-isomer.
Embodiment 2
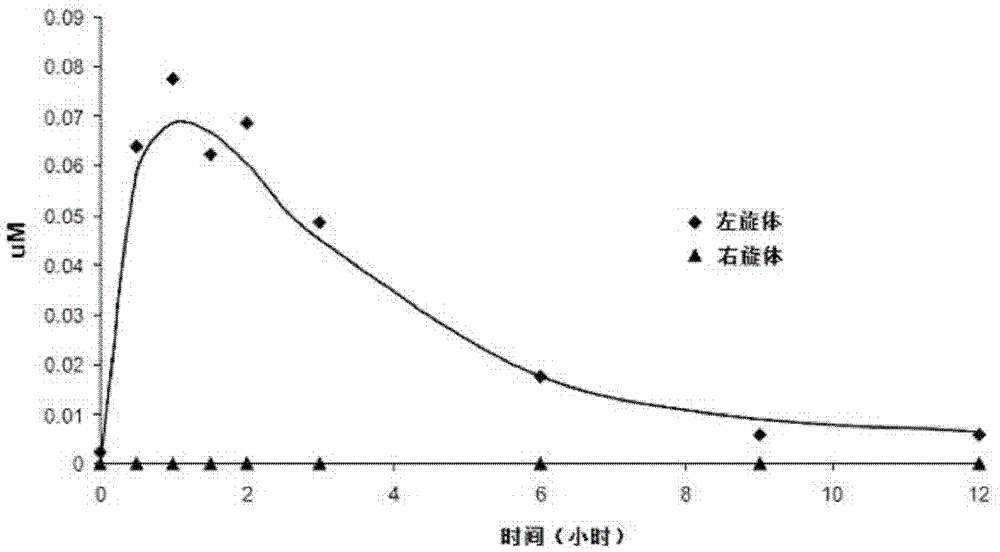
[0068] Analysis of L-isomers and D-isomers in Tablet Preparations
[0069] Determination of enantiomeric purity in levorotatory tablet formulations (lots 387I1100 and 116I0604) and determination of levorotatory in racemic tablet formulations (lot 157I0907) using isocratic reversed-phase high performance liquid chromatography with a chiral mobile phase The relative content of body and dextrorotatory body. The method includes the following steps:
[0070] Step 1: Analytical chiral mobile phase for HPLC. L-proline (1.86 g, 16 mmol, Sigma Aldrich product P-0380) and copper(II) nitrate hydrate (1.86 g, 8 mmol, Sigma Aldrich product 229636) were dissolved in 1 L of filtered and degassed HPLC grade water ( J.T.Baker product 4218-03). Using a pH meter, the pH of the solution was adjusted to 6.00 with 5N NaOH (approximately 2.5 ml).
[0071] Step 2: L-aminopterin standard preparation. A 1.0 mg / ml solution of L-aminopterin (Sigma Aldrich product A1784) was prepared in dimethylaceta...
Embodiment 3
[0083] Quantification of Total Aminopterin Isomers in Tablet Formulations
[0084] The total amount of aminopterin isomers in the pure levorotatory tablet formulations (Lot No. 387I1100 and 116I0604) and in the racemic tablet formulation (Lot No. 157I0907) were analyzed by reversed-phase HPLC gradient method. The method includes the following steps:
[0085] Step 1: Mobile Phase Preparation. Dissolve 100ml of 1.0M triethylammonium acetate buffer solution (1.0M TEAAC, Fluka product 90357) in 900ml of HPLC grade water to make 1L filtered and degassed 0.1M triethylammonium acetate buffer solution as solvent a. 1 L of filtered and degassed acetonitrile was used as solvent B.
[0086] Step 2: L-aminopterin standard. A certain amount (50 mg x 565.2 / 440.4) of L-aminopterin standard (disodium salt) was weighed (that is, the amount equivalent to 50 mg of free acid). Transfer the L-aminopterin standard to a 50 ml volumetric flask and dissolve to 50 ml with solvent A to provide a 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com