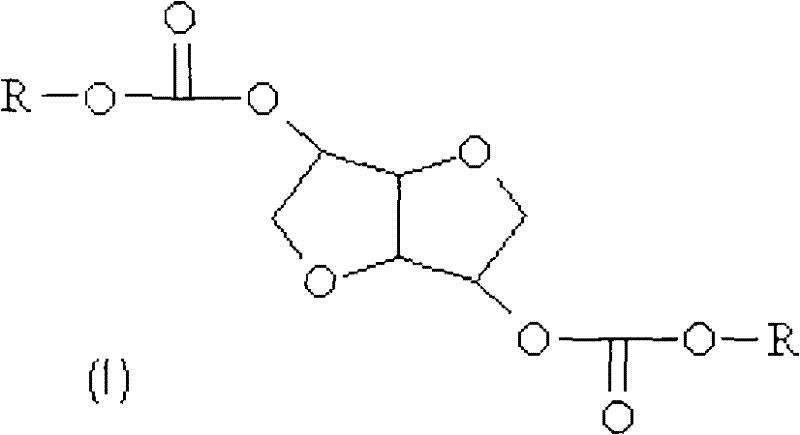
Method for preparing a dialkyl carbonate of dianhydrohexitol
A kind of technology of dianhydrohexitol and dialkyl carbonate, applied in the field of dialkyl carbonate for preparing dianhydrohexitol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0077] Example 1: Preparation of isosorbide bis(methyl carbonate) according to the invention
[0078]43 g of isosorbide (0.29 mol), then 1069 g of dimethyl carbonate (relative to isosorbide, = 40 molar equivalents) and 123 g of potassium carbonate were introduced into a reactor with a capacity of 1.5 liters by including heat A thermostatically controlled water bath of the exchange fluid heats the reactor, and the reactor is equipped with a paddle mechanical stirring system, a system for controlling the temperature of the reaction medium and a rectification column with a reflux head on top. The reaction mixture was heated at full reflux for one hour before starting to remove the methanol formed, at the end of which time the vapor temperature at the top of the column reached 64°C. Subsequent heating of the reaction medium is maintained at a temperature between 68°C and 95°C for 13 hours, at the end of which time the temperature of the vapor at the top of the column reaches 90°C ...
example 2
[0079] Example 2: Preparation of isosorbide bis(methyl carbonate) according to the invention
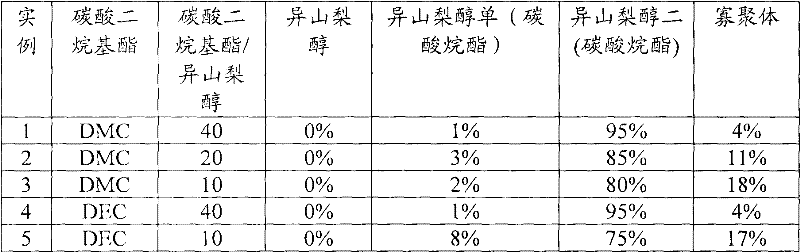
[0080] Example 1 was repeated with the only difference that instead of 40 equivalents, only 20 equivalents of dimethyl carbonate were used. The composition of the reaction medium obtained after removal of the catalyst particles is shown in Table 1 below.
example 3
[0081] Example 3: Preparation of isosorbide bis(methyl carbonate) according to the invention
[0082] Example 1 was repeated with the only difference that instead of 40 equivalents, only 10 equivalents of dimethyl carbonate were used. The composition of the reaction medium obtained after removal of the catalyst particles is shown in Table 1 below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com