Administration method for protecting bacteriophage activity and application thereof
A drug delivery method and phage technology, which are applied in the field of biomedicine and can solve problems such as no phage drug delivery method yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1. Isolation of bacteriophage (1) Preparation of the target bacterial suspension: Take the specimen of the infected site for isolation and pure culture as a target bacterial slant, add sterile water to wash the bacterial moss, and prepare a bacterial suspension. The medium and culture method used are all based on the culture of the target bacteria (the same below).
[0021] (2) Proliferation and culture of phages in the sample: In a three-fold concentrated liquid medium, filter-sterilized sewage samples were added in 1:2, and the target bacteria suspension was inoculated, so that the phages in the samples were cultured together with the target bacteria. The specific culture time is determined according to different bacteria, such as Escherichia coli and other bacteria culture for 12-24h; Helicobacter pylori culture for 5-7d (the same below).
[0022] (3) Preparation of phage lysate: after centrifuging the above mixed culture solution at 2500 r / min for 15 min, th...
Embodiment 2
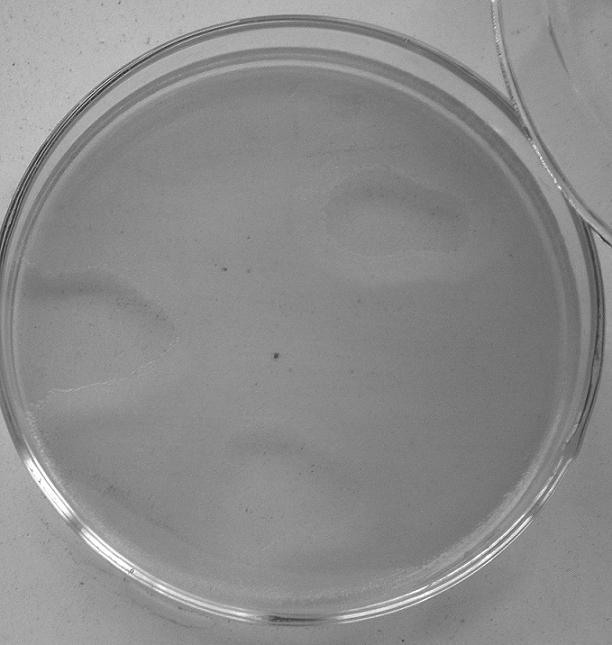
[0024] Example 2. Purification of bacteriophage (1) After the existence of bacteriophage was confirmed, a phage double-layer plate quantitative purification test was performed. The first tube uses an inoculation loop to take 2 loops of filtrate and add 0.2ml of suspension, the second tube of filtrate 5 loops add 0.2ml of bacterial suspension, the third tube of filtrate 10 loops add 0.2ml of bacterial suspension, and the fourth tube is made with 0.2ml of filtrate alone. Filtrate control, the fifth tube used 0.2ml bacterial suspension alone as bacterial control. Mix the tubes evenly and stand at 37°C for 15-30min.
[0025] (2) Take the upper layer of 0.8% agar medium, dissolve and cool to 48°C, add 4 ml to 0.2 ml of the above mixture of phage and bacteria, and mix immediately.
[0026] (3) and immediately pour it onto the bottom medium and spread it evenly. Incubate at 37°C for a certain period of time.
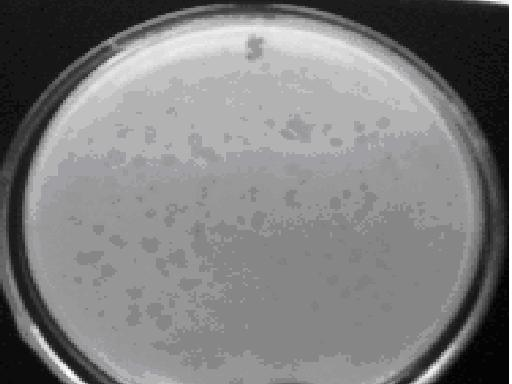
[0027] (4) The single plaques grown at this time are often inconsistent...
Embodiment 3
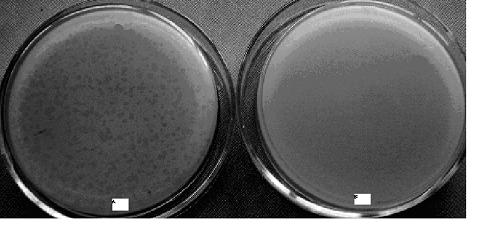
[0030] Example 3. Preparation of high-titer bacteriophage The phage obtained just after isolation and purification is often not high in titer and needs to be propagated. Mix the purified phage filtrate with the liquid medium at a ratio of 1:10, then add an appropriate amount of host bacterial suspension (it can be equal to or 1 / 2 of the amount of the phage filtrate), cultivate and multiply, and repeat the number of transplants. second, and finally filtered to obtain a high-titer phage product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com