Method for preparing epoxy chloropropane by micro-channel reactor
A technology of microchannel reactor and epichlorohydrin, which is applied in the direction of organic chemistry, can solve the problem of excessive waste water, and achieve the effects of simplified process, good environmental protection and safety, and easy scale-up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
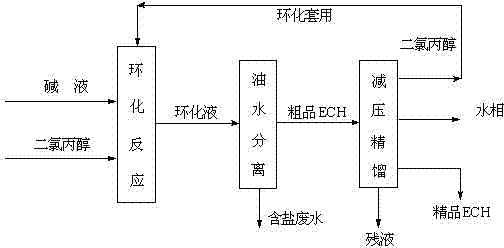
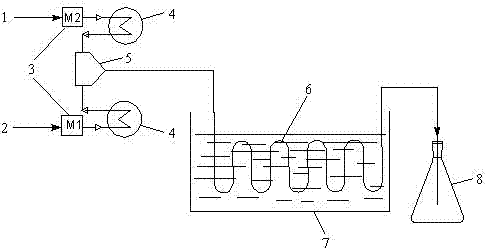
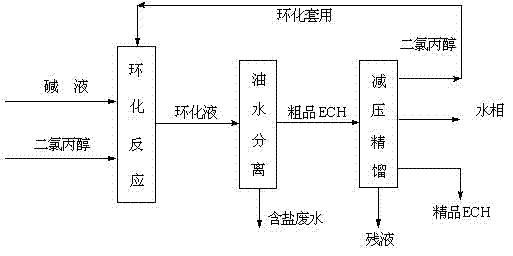
[0037] A microchannel reactor with an inner diameter of 0.2mm and a length of 15m was selected, and a NaOH solution with a mass fraction of 25% was selected for the cyclization reaction. Set the reaction temperature at 35°C, the residence time is 60s, and the feed molar ratio N[DCH]:N[NaOH]=1:1.4. Calculate the flow of the two materials according to the above molar ratio and residence time, and use a metering pump to first Dichloropropanol and NaOH are pumped into their respective preheaters for preheating through their respective pumps, and then the raw materials enter the micro-mixer for mixing, and finally the mixed material liquid reacts in the micro-channel, and the temperature of the constant temperature water tank is maintained at 35°C. After the reaction solution flows out, use 35% H 2 SO 4 The solution neutralizes it. The neutralized liquid is subjected to oil-water separation, and the oil phase is subjected to normal-pressure rectification to obtain refined epichl...
Embodiment 2
[0040] A microchannel reactor with an inner diameter of 0.4mm and a length of 10m was selected, and a NaOH solution with a mass fraction of 25% was selected for the cyclization reaction. Set the reaction temperature at 50°C, the residence time is 45s, the feed molar ratio N[DCH]:N[NaOH]=1:1.3, calculate the flow rate of the two materials according to the above molar ratio and residence time, and use the metering pump to first Dichloropropanol and NaOH are pumped into their respective preheaters for preheating through their respective pumps, and then the raw materials enter the micro-mixer for mixing, and finally the mixed material liquid reacts in the micro-channel, and the temperature of the constant temperature water tank is maintained at 50°C. After the reaction solution flows out, use 35% H 2 SO 4 The solution neutralizes it. The neutralized liquid is subjected to oil-water separation, and the oil phase is subjected to normal-pressure rectification to obtain refined epi...
Embodiment 3
[0043]A microchannel reactor with an inner diameter of 0.6mm and a length of 10m was selected, and a NaOH solution with a mass fraction of 25% was selected for the cyclization reaction. Set the reaction temperature at 65°C, the residence time is 30s, and the feed molar ratio N[DCH]:N[NaOH]=1:1.2. Calculate the flow rate of the two materials according to the above molar ratio and residence time, and use a metering pump to first Dichloropropanol and NaOH solution are pumped into their respective preheaters for preheating through their respective pumps, and then the raw materials enter the micro-mixer for mixing, and finally the mixed material liquid reacts in the micro-channel, and the temperature of the constant temperature water tank is maintained at 65°C. After the reaction solution flows out, use 35% H 2 SO 4 The solution neutralizes it. The neutralized liquid is subjected to oil-water separation, and the oil phase is subjected to normal-pressure rectification to obtain r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com