TAT-WNK102 transmembrane protein and application thereof
A TAT-WNK102, transmembrane protein technology, applied in the fields of peptide/protein components, recombinant DNA technology, medical preparations containing active ingredients, etc., can solve problems such as unclear downstream signal transduction pathways
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: Preparation of TAT-WNK102 fusion protein
[0018] The WNK102 protein corresponds to the amino acid sequence 308-409 of the human WNK-1 molecule, and is derived from a subclone using the full-length structure pEGFP-N1-hWNK-1 as a template. The production of the fusion protein TAT-WNK102 is to correctly insert the smallest translocation domain sequence (MRGSHHHHHHHGMARGYGRKKRRQRRRPQ) in the HIV1 Tat protein into the N-terminus of the corresponding WNK1 cDNA. The fusion protein was expressed by BL21 system in vitro and purified by nickel-affinity chromatography column according to the classical method.
Embodiment 2
[0019] Example 2: Culture of SD rat oligodendrocytes
[0020] The brains of SD neonatal rats were taken, and the bilateral cerebral cortex was separated, D. Wash with Hanks solution, pipette to make cell suspension, filter with 200-mesh cell mesh, centrifuge at 180×g for 5 min, discard supernatant, add high-glucose DMEM culture medium containing 10% fetal bovine serum to resuspend cells, dilute to (1.0 -2.0) *106 density planted in 0.025% poly-lysine pretreated 75c㎡ culture flask and cultured in 5% CO2 for 8-11 days, after the cells were confluent and formed obvious stratification, OPCs shaking separation and purification began (the culture flask Place on a constant temperature air bath vibrating shaker, 37 degrees, 150r / min, shake for 2h to remove microglial cells, etc. D. Wash with Hanks solution, change the liquid; 5% CO2 incubator balance for 2h; 230r / min, 16-18h , 280r / min, 1-2h; collect the cell suspension, centrifuge at 400×g for 5 min, resuspend the cells in high-gluc...
Embodiment 3
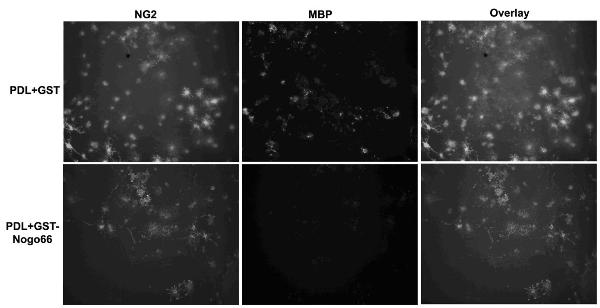
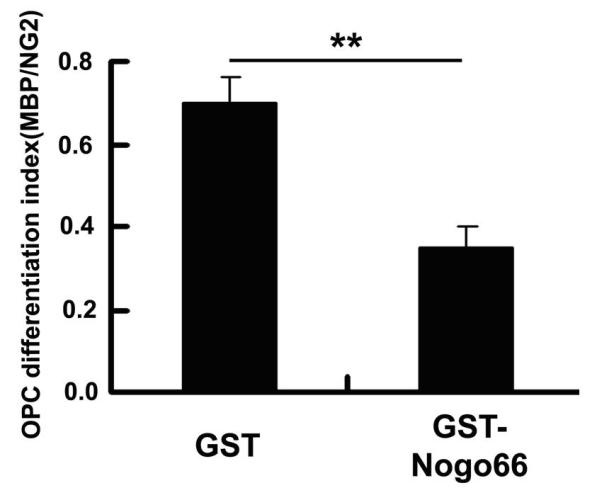
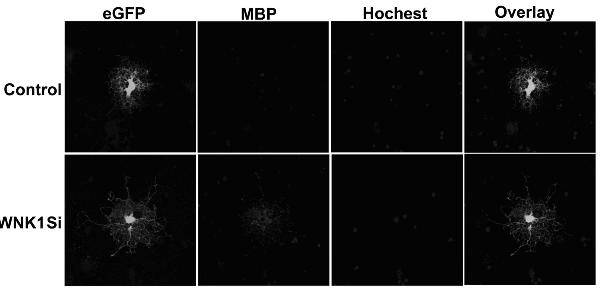
[0021] Embodiment 3: The SD rat oligodendrocytes purified in Example 2 were inoculated on a 35mM culture plate coated with GST-Nogo66 protein or GST protein in advance, and replaced with fresh Neurobasal containing 2% B27 after three hours -A medium (Gibco), . After cultured for 5 days, the cells were immunohistochemically performed with anti-MBP antibody and observed and photographed under a confocal microscope. See figure 1 and figure 2 , using Nogo66 protein as a substrate to culture primary cultured rat OPC cells will indeed inhibit the differentiation and maturation of OPC cells in its differentiation medium. The number of OPC differentiated cells grown on Nogo66 matrix was significantly reduced. figure 2 for figure 1 Statistical analysis of the proportion of differentiated cells. Data are from three independent experiments and expressed as mean ± standard error. Comparison by Student's-t-test, p<0.001.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com