Cloning vector
A carrier and drug resistance technology, which is applied in the field of PCR product cloning vectors, can solve the problem of not being able to control the directionality of the target gene, and achieve the effect of simplifying the preparation operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] (1) Construction of the vector
[0078] The vector of the present invention is constructed by the following method. First, the pUC19 vector (manufactured by Takara Bio Co., Ltd.) was digested with restriction enzymes Nde I and PvuII (both manufactured by Takara Bio Co., Ltd.). This was followed by blunt endization and BAP treatment. The DNA fragment thus obtained is purified by a conventional method. Next, a double-stranded DNA having a nucleic acid sequence from the 999th base to the 213th base in the nucleic acid sequence described in SEQ ID NO: 5 of the Sequence Listing is synthesized. Next, this double-stranded DNA was inserted into pUC 19 DNA cut with restriction enzymes Nde I and Pvu II. The plasmid DNA thus constructed was named pUC19-Kana DNA.
[0079] Inverse PCR was performed using the above pUC19-Kana DNA as a template. First, AMP-F primers and AMP-R primers each having the nucleotide sequences described in SEQ ID NOs: 3 and 4 of the Sequence Listing wer...
Embodiment 2
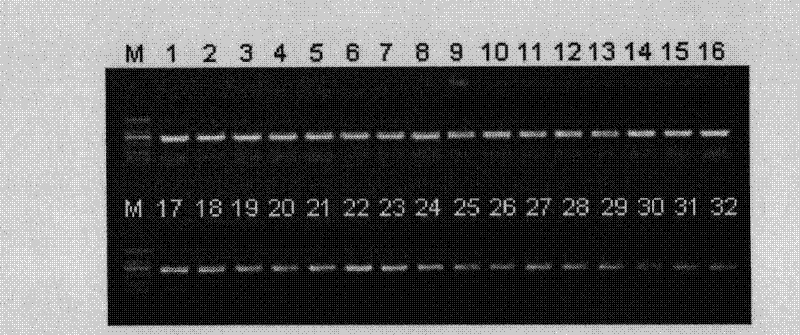
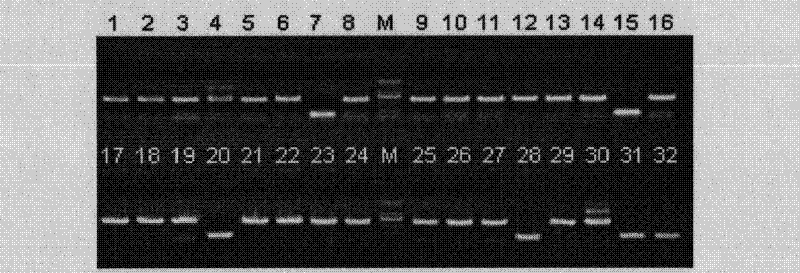
[0084] Example 2 Regarding the pUC19-blaΔW290-T DNA prepared in Example 1, PCR fragments of various chain lengths were inserted by the TA cloning method to discuss the efficiency of obtaining positive clones.
[0085] (1) Discussion on the efficiency of obtaining positive clones of 500bp PCR fragments
[0086] A 500bp-F primer and a 500bp-R primer having the nucleic acid sequences described in SEQ ID NOs: 6 and 7 of the Sequence Listing, respectively, were synthesized. Next, PCR was performed using these primers using Escherichia coli JM 109 genomic DNA as a template. PCR was performed in the following reaction composition. That is, 50 μL of the reaction solution contained 25 μL of 2×Ex Taq Premix (manufactured by Takara Bio Co., Ltd.), 20 pmol of each of the aforementioned primers, and 50 ng of template DNA. The PCR conditions were 1 minute at 94°C, (15 seconds at 98°C, 30 seconds at 59°C, and 30 seconds at 72°C) as one cycle, followed by 30 cycles of reaction, followed by ...
Embodiment 3
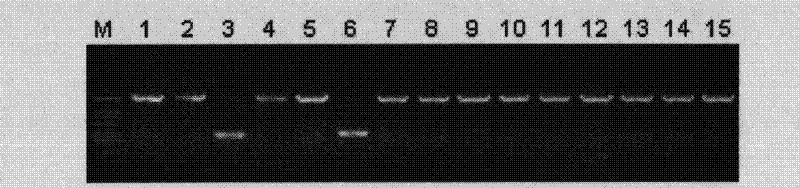
[0097] The relationship between the vectors and inserts of the present invention is discussed. In this example, the 500 bp PCR amplified fragment and the 2 kbp PCR amplified fragment prepared in the above-mentioned Example 2(1) and Example 2(3) were used. Six kinds of 10 μL reaction solutions were prepared, which respectively contained 25 ng, 50 ng or 100 ng of the vector of the present invention relative to 100 ng of the 500 bp or 2 kbp amplified fragment, and DNA Ligation kit ver. These solutions were kept at 16°C for 1 hour. Transformation and culture conditions were the same as in Example 2(1). As a result, in the case of inserting a 500 bp PCR fragment, it was confirmed that the number of clones obtained was increased as the vector concentration increased. On the other hand, when the 2 kbp PCR fragment was inserted, it was confirmed that the maximum number of clones was obtained when the vector amount was 50 ng.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com