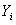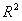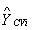Method by utilizing near infrared spectrum to measure content of multiple ingredients of complex sophora flavescens injection during percolation process
A technology of near-infrared spectroscopy and Sophora flavescens injection, which is applied in the field of quality control of traditional Chinese medicine production, can solve the problems of not meeting the requirements of rapid real-time monitoring, hindering the industrialization development of traditional Chinese medicine, low accuracy, etc., and achieves shortening analysis and measurement time and linear relationship. Good and accurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Collection and preprocessing of calibration sample sets.
[0041] Take different batches of Sophora flavescens and Poria cocos medicinal materials, grind them into coarse powders of 0.2mm-0.81mm, weigh 70g of Sophora flavescens powder and 30g of Poria cocos medicine powder, mix them, add 1200ml of 1% acetic acid aqueous solution, soak for 24 hours, and pack the powder into Enter the percolation device, compact and start percolation. The filtrate is 600ml of 1% acetic acid aqueous solution, the percolation time is 24h, and the flow rate is 0.5ml / min. During the percolation process, samples were taken every 15 minutes to ensure that the content distribution of each component of the sample had a certain range, and a total of 80 samples were collected. The samples collected above were first filtered with four layers of filter paper, then centrifuged at 1000rmp for 10min, and the supernatant was taken for content determination and spectrum collection.
[0042] 2. Calibr...
Embodiment 2
[0065] The steps of the method are the same as those in Example 1, except that a calibration model for oxysophocarpine is established.
[0066] 1. The collection and pretreatment of the calibration sample set are the same as in Example 1.
[0067] 2. The determination of the content of oxysophocarpine in the calibration sample set is the same as in Example 1.
[0068] The measured distribution range of oxysophocarpine content in the calibration sample set is 0.026-0.522 mg / ml.
[0069] 3. The NIR spectrum data collection of the calibration sample set is the same as in Example 1.
[0070] 4. The establishment of the oxidized sophocarpine calibration model is the same as in Example 1.
[0071] Select SNV Pretreatment, 5388-6572cm -1 The performance of the PLS regression model established by Band is the best, that is, the maximum correlation coefficient is 0.980, the minimum RMSECV is 0.327 mg / ml, and the optimal number of factors is 3. The correlation between the reference v...
Embodiment 3
[0078] The steps of the method are the same as those in Example 1, except for the determination of the content of pterostaloside and the establishment of the calibration model.
[0079] 1. The collection and pretreatment of the calibration sample set are the same as in Example 1.
[0080] 2. Calibrate the determination method of the content of pteroside in the sample set.
[0081] HPLC chromatographic conditions: Phenomenex Luna C18 chromatographic column (250mm×4.6mm, 5um); mobile phase: acetonitrile:water=30:70; flow rate: 1.0mL / min; detection wavelength 310nm; column temperature 35°C.
[0082] The distribution range of the content of trilobalin in the calibration sample set was 0.002-0.041 mg / ml.
[0083] 3. The NIR spectrum data collection of the calibration sample set is the same as in Example 1.
[0084] 4. The establishment of the pterostaloside calibration model is the same as in Example 1.
[0085] Select SNV Pretreatment, 5388-6572cm -1 The performance of the PLS...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com