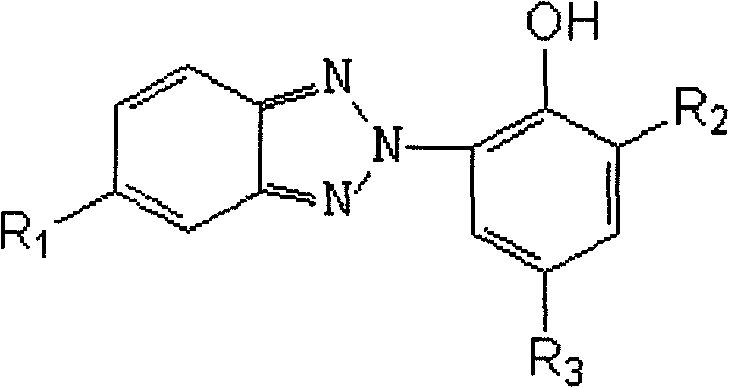
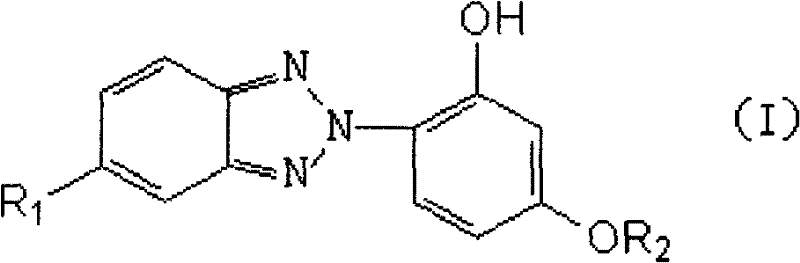
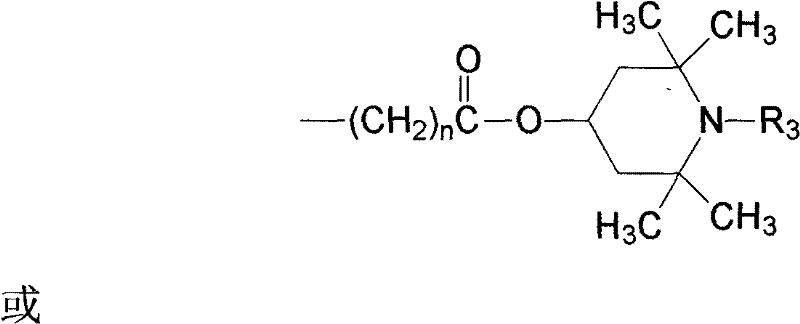
Novel hindered amine group-contained benzotriazole light stabilizer
A technology of benzotriazole and hindered amine, applied in the field of benzotriazole light stabilizer, can solve the problems of unsatisfactory ultraviolet absorption intensity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064]Add 10.0g (0.03195mol) 2-[2-hydroxy-4-ethoxycarbonylmethoxyphenyl]-2H-benzotriazole, 10.0 g (0.0637mol) 2,2,6,6-tetramethylpiperidinol, 0.2g dibutyltin oxide and 50mL xylene were reacted at 140°C for 6 hours. After the reaction was completed, the temperature was lowered, filtered, dried, and then recrystallized with ethyl acetate to obtain a white solid, namely 2-[2-hydroxyl-4-(2,2,6,6-tetramethylpiperidine-4-oxyl) Carbonylmethoxyphenyl]-2H-benzotriazole (compound II) 8.1g, yield 59.3%, high performance liquid chromatography (HPLC) purity 99.2%, melting point 160.8-161.0°C.
[0065] Elemental Analysis Results:
[0066]
Embodiment 2
[0068] Add 10.0 g (0.0288 mol) of 5-chloro-2-[2-hydroxy-4-ethoxycarbonylmethoxyphenyl]-2H-benzo Triazole, 10.0 g (0.0637 mol) of 2,2,6,6-tetramethylpiperidinol, 0.2 g of dibutyltin oxide and 50 mL of solvent oil were reacted at 140° C. for 7 hours. After the reaction was completed, the temperature was lowered, filtered, dried, and recrystallized from ethyl acetate to obtain a white solid, namely 5-chloro-2-[2-hydroxyl-4-(2,2,6,6-tetramethylpiperidine-4 -Oxy)carbonylmethoxyphenyl]-2H-benzotriazole (compound III) 7.7g, yield 57.8%, HPLC purity 99.1%, melting point 167.1-167.4°C.
[0069] Elemental Analysis Results:
[0070]
Embodiment 3
[0072] Add 10.0g (0.03195mol) 2-[2-hydroxy-4-ethoxycarbonylmethoxyphenyl]-2H-benzotriazole, 10.0 g (0.0585 mol) of 1,2,2,6,6-pentamethylpiperidinol, 0.2 g of tetrabutyl titanate and 50 mL of xylene were reacted at 140° C. for 6 hours. After the reaction was completed, the temperature was lowered, filtered, dried, and then recrystallized with ethyl acetate to obtain a white solid, namely 2-[2-hydroxyl-4-(1,2,2,6,6-pentamethylpiperidine-4-oxo yl)carbonylmethoxyphenyl]-2H-benzotriazole (compound IV) 7.8g, yield 55.4%, HPLC purity 99.3%, melting point 114.3-114.8°C.
[0073] Elemental Analysis Results:
[0074]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com