Chinese medicine composite and preparation method and medicinal preparation thereof
A technology of traditional Chinese medicine preparations and compositions, which is applied in the field of traditional Chinese medicine compositions and their preparation methods and preparations, which can solve the problems of low drug efficacy, failure to meet the treatment requirements of cancer patients, and failure of anti-tumor methods to suppress tumors, and achieve recovery. and immune function, enhance the activity, enhance the effect of immune function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: prepare Chinese medicine composition of the present invention
[0027] 1. Preparation method
[0028] Take 20g of red lucidum and grind it into a powder with a diameter of 2000μm, add water to soak for 12 hours, decoct for 3 hours, then filter, add water to the filter residue and decoct for 3 hours, then filter, combine the filtrates obtained twice, and concentrate at 80°C to a relative density of 1.36 , and then dried and crushed into a powder with a diameter of 180 μm to obtain 1.7 g of Ganoderma lucidum extract, which was mixed with 0.27 g of ginsenoside Rh2.
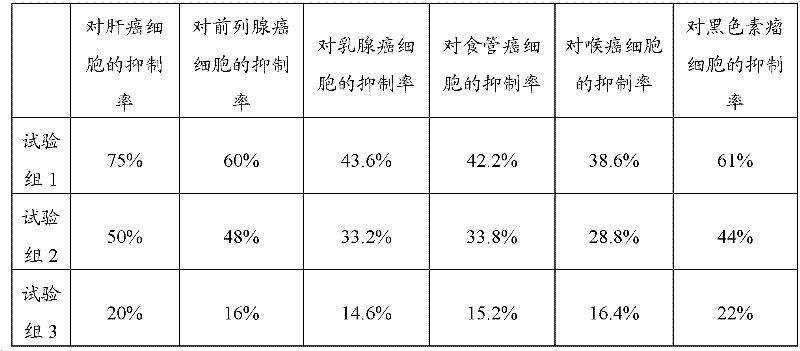
[0029] 2. Tumor suppression test
[0030] Tumor cells: human liver cancer cell line BeI-7404, prostate cancer cell line PC-3M, human breast cancer cell line MCF-7, esophageal cancer cell line Eca-190, human laryngeal cancer cell line Hep-2, mouse melanoma cells Strain B16 was purchased from Dalian Institute of Chemical Physics;
[0031] Tested drug and grouping: the traditional Chinese medicin...
Embodiment
[0041] 1. Preparation method
[0042] Take 24g flat-covered Ganoderma lucidum and crush it into a powder with a diameter of 2000μm, add water to soak for 12 hours, decoct for 3 hours, then filter, add water to the filter residue and decoct for 3 hours, then filter, combine the filtrates obtained twice, and concentrate at 80°C to relative density 1.38, then dried and crushed into a powder with a diameter of 180 μm to obtain 1.8 g of Ganoderma lucidum extract, which was mixed with 0.31 g of ginsenoside Rh2 by the incremental dilution method.
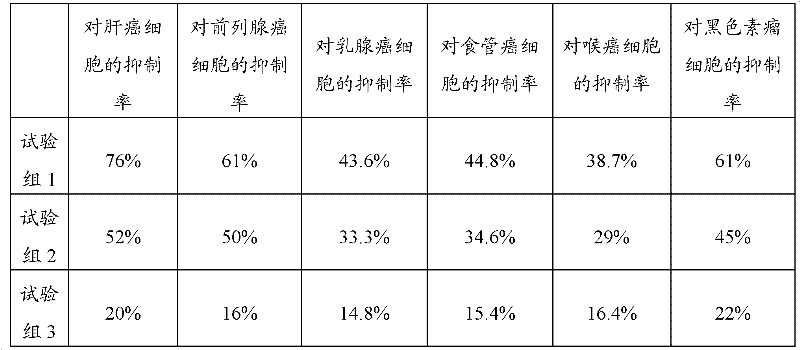
[0043] 2. Tumor suppression test
[0044] Tumor cells: human liver cancer cell line BeI-7404, prostate cancer cell line PC-3M, human breast cancer cell line MCF-7, esophageal cancer cell line Eca-190, human laryngeal cancer cell line Hep-2, mouse melanoma cells Strain B16 was purchased from Dalian Institute of Chemical Physics;
[0045]Tested drug and grouping: the traditional Chinese medicine composition prepared according to the prepar...
Embodiment 3
[0055] 1. Preparation method
[0056] Take 16g of red lucidum and crush it into a powder with a diameter of 2000μm, add water to soak for 12 hours, decoct for 3 hours and then filter, add water to the filter residue and decoct for 3 hours again, then filter, combine the filtrate obtained twice, and concentrate at 80°C to a relative density of 1.35 , and then dried and crushed into a powder with a diameter of 180 μm to obtain 1.3 g of Ganoderma lucidum extract, which was mixed with 0.23 g of ginsenoside Rh2 by the incremental dilution method.
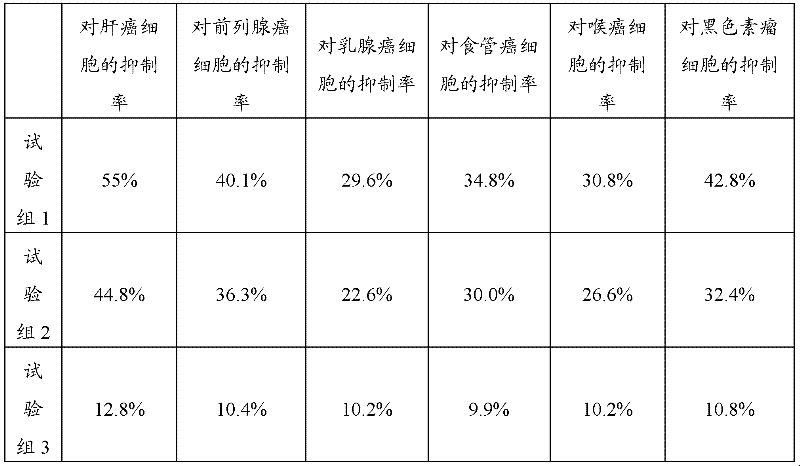
[0057] 2. Tumor suppression test
[0058] Tumor cells: human liver cancer cell line BeI-7404, prostate cancer cell line PC-3M, human breast cancer cell line MCF-7, esophageal cancer cell line Eca-190, human laryngeal cancer cell line Hep-2, mouse melanoma cells Strain B16 was purchased from Dalian Institute of Chemical Physics;
[0059] Tested drug and grouping: the traditional Chinese medicine composition prepared according to the pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com