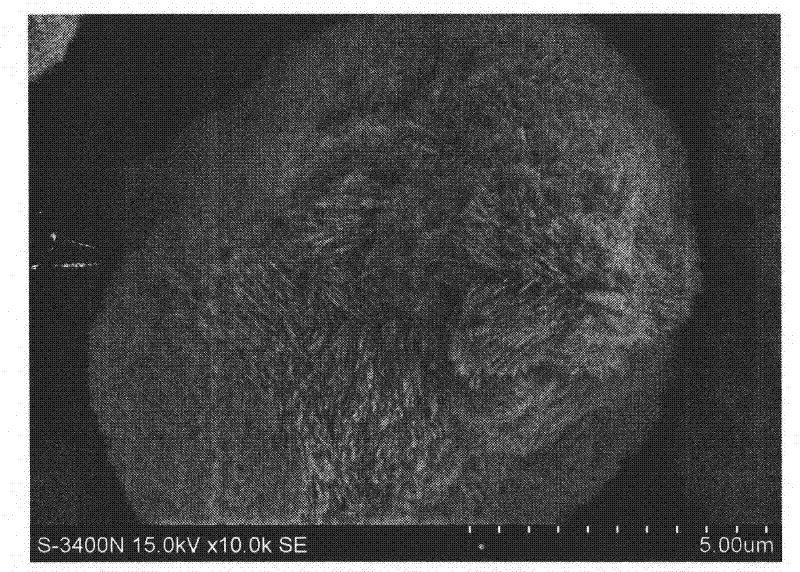
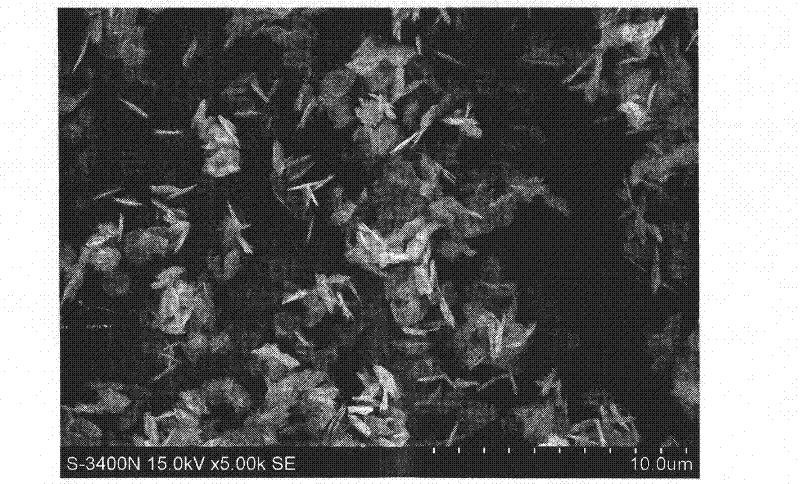CuO loaded CeO2 catalyst for CO preferential oxidation
A catalyst and carrier technology, applied in the field of CuO supported CeO2 catalyst, can solve the problems of high price, catalyst deactivation, large influence on catalyst activity, etc., achieve good CO conversion and selectivity, simple and easy preparation process, and temperature window. wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A CuO-supported CeO for preferential oxidation of CO 2 The preparation method of catalyst, its step comprises:
[0028] 1) Take 0.02mol Cu(NO 3 ) 2 Solution 600ml, spare;
[0029] 2) The configuration of the mixed solution of ammonia water and PEG: according to the Cu(NO 3 ) 2 The molar ratio of the solution is 8:1. Take 0.16mol of concentrated ammonia water, that is, 24.6ml, then add 24.6ml of deionized water according to the volume ratio of ammonia water to water is 1:1, and then add 3g of polyethylene glycol with Mw=6000 to the solution. Alcohol, sonicated until completely dissolved, made into a mixed solution, and set aside;
[0030] 3) the Cu(NO in step 1) 3 ) 2 The solution was heated to 30°C, and then the mixed solution in step 2) was added dropwise to the stirred Cu(NO 3 ) 2 in solution;
[0031] 4) After the dropwise addition, the Cu(NO 3 ) 2 The mixture was heated to 80°C and stirred for 12 hours at the same time, then stood at 20°C for 12 hours, f...
Embodiment 2
[0035] A CuO-supported CeO for the preferential oxidation of CO 2 The preparation method of catalyst, its step comprises:
[0036]1) Take 0.02mol Cu(NO 3 ) 2 Solution 600ml, spare;
[0037] 2) The configuration of the mixed solution of ammonia water and PEG: according to the Cu(NO 3 ) 2 The molar ratio of the solution is 8:1. Take 0.16mol of concentrated ammonia water, that is, 24.6ml, then add 24.6ml of deionized water according to the volume ratio of ammonia water to water is 1:1, and then add 5g of polyethylene glycol with Mw=6000 to the solution. Alcohol, sonicated until completely dissolved, made into a mixed solution, and set aside;
[0038] 3) the Cu(NO in step 1) 3 ) 3 The solution was heated to 30°C, and then the mixed solution in step 2) was added dropwise to the stirred Cu(NO 3 ) 2 in solution;
[0039] 4) After the dropwise addition, the Cu(NO 3 ) 2 The mixture was heated to 80°C and stirred for 12 hours at the same time, then stood at 20°C for 12 hours...
Embodiment 3
[0043] A CuO-supported CeO for the preferential oxidation of CO 2 The preparation method of catalyst, its step comprises:
[0044] 1) Take 0.02mol Cu(NO 3 ) 2 Solution 600ml, spare;
[0045] 2) The configuration of the mixed solution of ammonia water and PEG: according to the Cu(NO 3 ) 2 The molar ratio of the solution is 8:1. Take 0.16mol of concentrated ammonia water, that is, 24.6ml, and then add 24.6ml of deionized water according to the volume ratio of ammonia water to water is 1:1, and then add 6gMw=6000 polyethylene glycol to the solution. Alcohol, sonicated until completely dissolved, made into a mixed solution, and set aside;
[0046] 3) the Cu(NO in step 1) 3 ) 2 The solution was heated to 30°C, and then the mixed solution in step 2) was added dropwise to the stirred Cu(NO 3 ) 2 in solution;
[0047] 4) After the dropwise addition, the Cu(NO 3 ) 2 The mixture was heated to 80°C and stirred for 12 hours at the same time, then stood at 20°C for 12 hours, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com