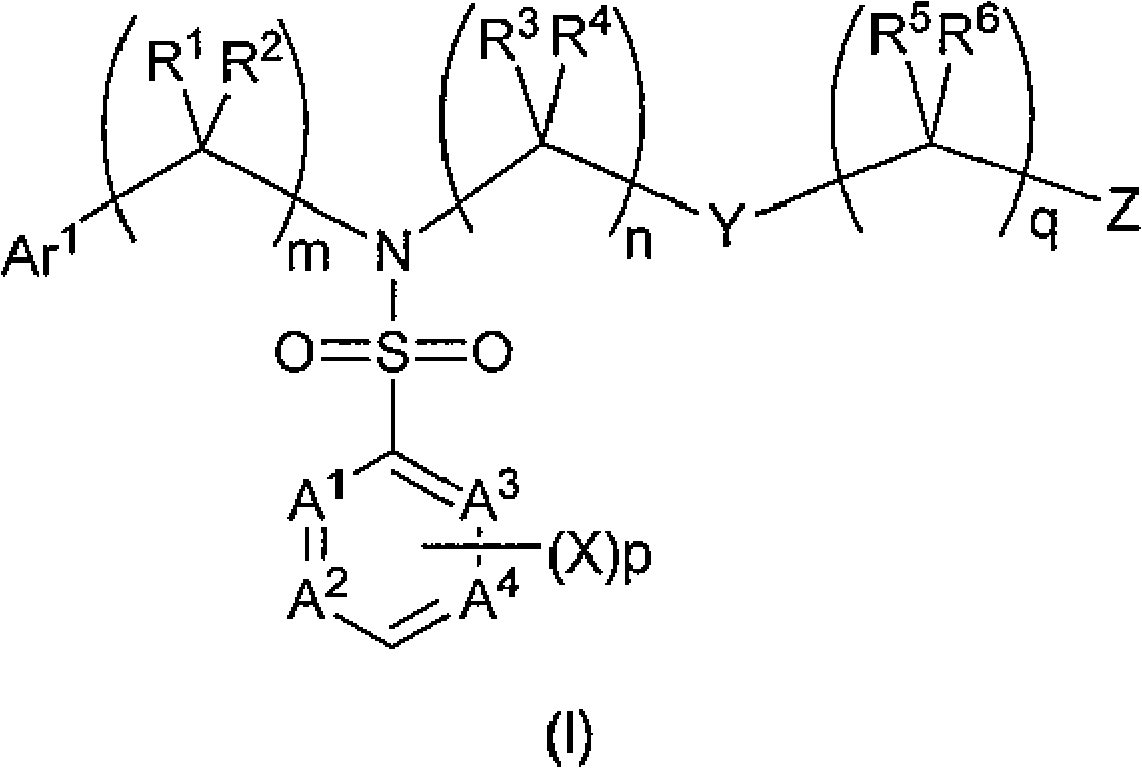
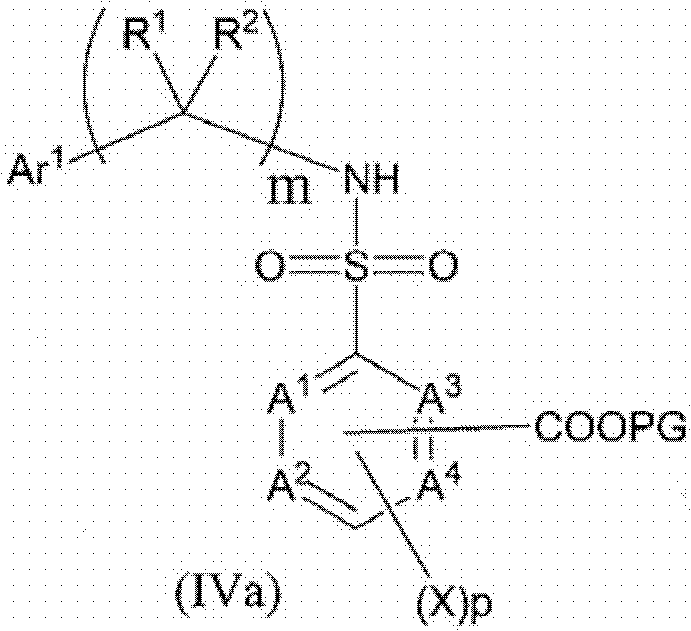
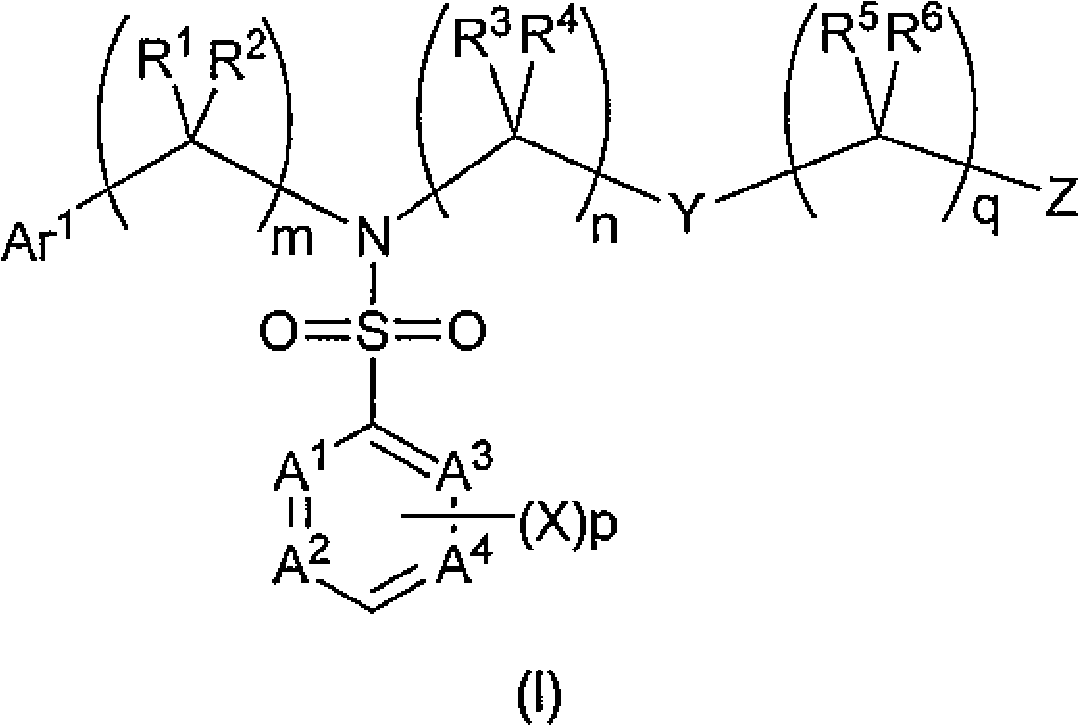
Sulfamoyl benzoic acid derivatives as trpm8 antagonists
An amino and receptor antagonism technology, which can be used in anti-inflammatory agents, drug combinations, anti-tumor drugs, etc., to solve the problems of sensory organ neurotoxicity in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0447] 4-(N-Benzyl-N-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)sulfamoyl)benzoic acid
[0448] Step-1: Methyl 4-(N-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)sulfamoyl)benzoate salt
[0449] To a solution of 3-chloro-5-(trifluoromethyl)pyridin-2-amine (1.0 g, 5.1 mmol) in pyridine (5 mL) was added methyl 4-(chlorosulfonyl)benzoate (1.3 g, 5.6 mmol), and the mixture was refluxed for 14 hours. The mixture was concentrated under reduced pressure. The residue was dissolved in DCM. Next, with 2M aqueous HCl and saturated NaHCO 3 Wash the organic layer and make it in MgSO 4 Dry on top. Filtration was performed to make the solvent and MgSO 4 After the layers were separated, the solvent was removed under reduced pressure to obtain 550 mg (27% yield) of methyl 4-(N-(3-chloro-5-(trifluoromethyl)pyridin-2-yl) as a dark solid Sulfamoyl) benzoate was used in the next step without further purification;
[0450] 1 H-NMR (300MHz, DMSO-d 6 )δ8.15-8.05 (2H, m), 8.05-7.95 (2H, m),...
Embodiment 2
[0461] 4-(N-Benzyl-N-(3-(trifluoromethyl)pyridin-2-yl)sulfamoyl)benzoic acid
[0462] Step-1: N-Benzyl-3-(trifluoromethyl)pyridin-2-amine
[0463] At room temperature, in 2-chloro-3-(trifluoromethyl)pyridine (500mg, 2.8mmol) and K 2 CO 3 (1.9g, 13.8mmol) in DMF (5mL) was added benzylamine (0.9g, 8.3mmol), and the mixture was stirred at 110°C for 16 hours. The reaction was quenched with water, and the product was extracted with (EtOAc / toluene=4 / 1). Next, the organic layer was washed with brine and placed in Na 2 SO 4 Dry on top. Filtration is performed to make the solvent and Na 2 SO 4 After separation, the solvent was removed under reduced pressure to obtain a residue, which was applied to an amino-silica gel chromatography column and eluted with hexane / EtOAc=19 / 1 to obtain 363 mg (52% yield) of the title compound as a white solid ;
[0464] 1 H-NMR (300MHz, CDCl 3 )δ8.28 (1H, d, J = 4.8Hz), 7.67 (1H, d, J = 7.3Hz), 7.40-7.30 (5H, m), 6.65 (1H, dd, J = 7.3, 4.8H...
Embodiment 3
[0469] N-Benzyl-N-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)benzenesulfonamide
[0470] Step-1: N-(3-Chloro-5-(trifluoromethyl)pyridin-2-yl)benzenesulfonamide
[0471] As in step-1 of Example 1, it was prepared from benzenesulfonyl chloride;
[0472] 1 H-NMR (CDCl 3 , 300MHz) δ8.41 (1H, s), 8.18 (2H, d, J=7.3Hz), 8.00-7.80 (2H, m), 7.70-7.50 (3H, m);
[0473] LC-MS (Method A) m / z: M+1 obs 336.9, tR = 2.97 min.
[0474] Step-2: N-Benzyl-N-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)benzenesulfonamide
[0475] As in step-2 of Example 1, it was prepared from N-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)benzenesulfonamide (step-1 of Example 3);
[0476] 1 H-NMR (300MHz, CDCl 3 )δ8.52 (1H, d, J = 1.5Hz), 7.93 (1H, d, J = 2.2Hz), 7.90-7.80 (2H, m), 7.68 (1H, m), 7.60-7.52 (2H, m ), 7.37 (1H, m), 7.25-7.10 (4H, m), 4.69 (2H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com