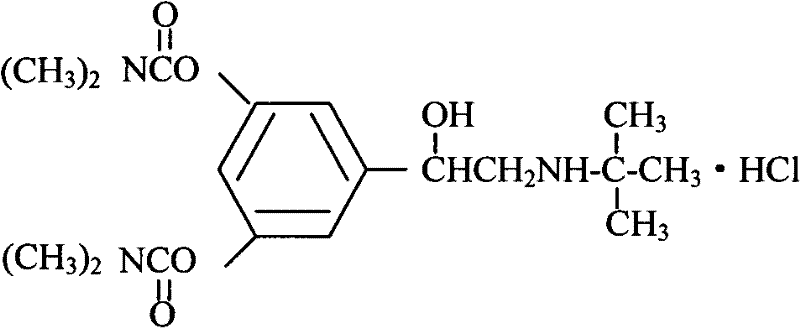
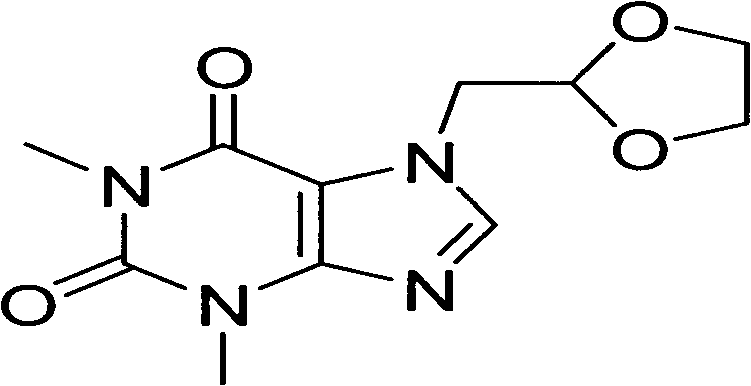
Bambutero hydrochloride and doxofylline-contained compound preparation and preparation method thereof
A technology for bambuterol hydrochloride and doxofylline, which is applied in the directions of ester active ingredients, heterocyclic compound active ingredients, respiratory system diseases, etc., can solve problems such as less side effects, and achieves convenient administration, reasonable formula, and preparation technology. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0041] The compound tablet of bambuterol hydrochloride and doxofylline comprises main ingredients and auxiliary materials, and is characterized in that it is formulated according to the following weight percentages: main ingredients: 42%, auxiliary materials: 58%.
[0042]
[0043] Among them, corn starch and lactose are fillers; gelatin and talc are effervescent agents; magnesium stearate is a lubricant.
[0044] The preparation method of bambuterol hydrochloride and doxofylline compound tablet of the present invention, comprises raw material pulverizing, weighing, the direct tabletting operation after mixing, is characterized in that concrete process step is as follows:
[0045] Step 1: Pulverize bambuterol hydrochloride, doxofylline and the above-mentioned various auxiliary materials respectively, then pass through a 100-mesh sieve, and store them separately for later use;
[0046] Step 2: Weigh each component according to the above-mentioned prescription amount, and mix...
example 2
[0049] The compound tablet of bambuterol hydrochloride and doxofylline includes main ingredients and auxiliary materials, and is characterized in that it is formulated according to the following weight percentages: main ingredients: 84%, auxiliary materials: 16%.
[0050]
[0051] Among them, corn starch and lactose are fillers; gelatin and talc are effervescent agents; magnesium stearate is a lubricant.
[0052] The preparation method of bambuterol hydrochloride and doxofylline compound tablet of the present invention, comprises raw material pulverizing, weighing, the direct tabletting operation after mixing, is characterized in that concrete process step is as follows:
[0053] Step 1: Pulverize bambuterol hydrochloride, doxofylline and the above-mentioned various auxiliary materials respectively, then pass through a 100-mesh sieve, and store them separately for later use;
[0054] Step 2: Weigh each component according to the above-mentioned prescription amount, and mix ...
Embodiment 3
[0057] The compound syrup of bambuterol hydrochloride and doxofylline includes main ingredients and auxiliary materials, and is characterized in that it is formulated according to the following weight percentages: main ingredients: 1.26%, auxiliary materials: 98.74%.
[0058] Compound syrup of the present invention is formulated from the raw materials of following weight ratio:
[0059]
[0060] Among them, liquid glucose and sucrose are sweet additives; vitamin C is an antioxidant; sodium metabisulfite is a preservative; edetate disodium is a complexing agent; purified water is a diluent.
[0061]The preparation method of bambuterol hydrochloride and doxofylline compound syrup of the present invention comprises the steps of pulverizing raw materials, weighing, mixing, stirring, filtering and sterilizing, and is characterized in that the specific process steps are as follows:
[0062] Step 1: Pulverize bambuterol hydrochloride, doxofylline and the above-mentioned various au...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com