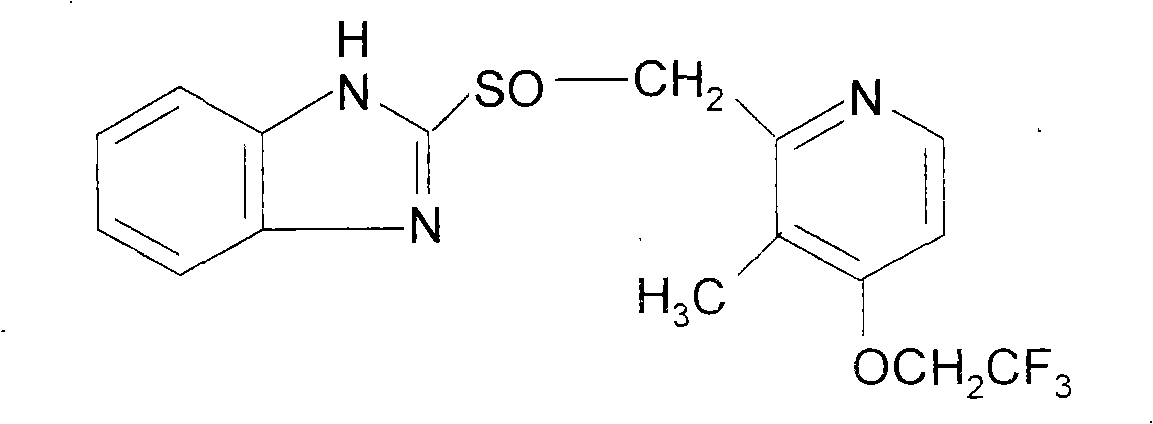
Prescription and preparation method of injection lansoprazole
A lansoprazole prescription and lansoprazole technology, applied in the field of injections, can solve the problems of complex preparation process of solid preparations, increased drug side effects, high production costs, etc., to improve drug bioavailability, reduce production costs, and protect activity The effect of ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Lansoprazole 30.0g
[0021] Mannitol 90.0g
[0023]
[0024] Made 1000 pieces
[0025] Take by weighing prescription quantity mannitol, join in the water for injection of preparation full amount 85% (water temperature 30~40 ℃), stir and dissolve, add lansoprazole, adjust to PH11.0 with 1mol / L sodium hydroxide under stirring, supplement Add water for injection to the full amount, add medicinal charcoal, stir and absorb at room temperature, filter to remove charcoal; take the filtrate for filling, put the filled product on the partition of the freeze-drying box, pre-freeze at -33°C for 2.5 hours, and vacuum to a vacuum When the temperature reaches 15pa, raise the temperature at 2°C / h until the product temperature reaches above 5°C, and then set the partition temperature to 35°C for secondary drying. The product temperature reaches above 30°C, equilibrate for 4 hours, and press the plug to obtain Lansol for Injec...
Embodiment 2
[0027] Lansoprazole 30.0g
[0028] Mannitol 150.0g
[0029] Sodium hydroxide 3.4g
[0030]
[0031] Made 1000 pieces
[0032] Take by weighing prescription quantity mannitol, join in the water for injection of preparation full amount 85% (water temperature 30~40 ℃), stir and dissolve, add lansoprazole, adjust to PH12.0 with 1mol / L sodium hydroxide under stirring, supplement Add water for injection to the full amount, add medicinal charcoal, stir and absorb at room temperature, filter to remove charcoal; take the filtrate for filling, put the filled product on the partition of the freeze-drying box, pre-freeze at -33°C for 2.5 hours, and vacuum to a vacuum When the temperature reaches 15pa, raise the temperature at 2°C / h until the product temperature reaches above 5°C, and then set the partition temperature to 35°C for secondary drying. The product temperature reaches above 30°C, equilibrate for 4 hours, and press the plug to obtain Lansol for Inje...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com