High-efficiency stabilizing agent for hard-soluble medicine nanometer system
A technology of insoluble drugs and stabilizers, applied in the field of medicine, can solve the problems of complicated prescription composition and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Itraconazole Nanosuspension
[0016] Itraconazole 1.0g
[0017] Poloxamer 188 0.5g
[0018] Chitosan 0.2g
[0019] Distilled water 100ml
[0020] Preparation Process:
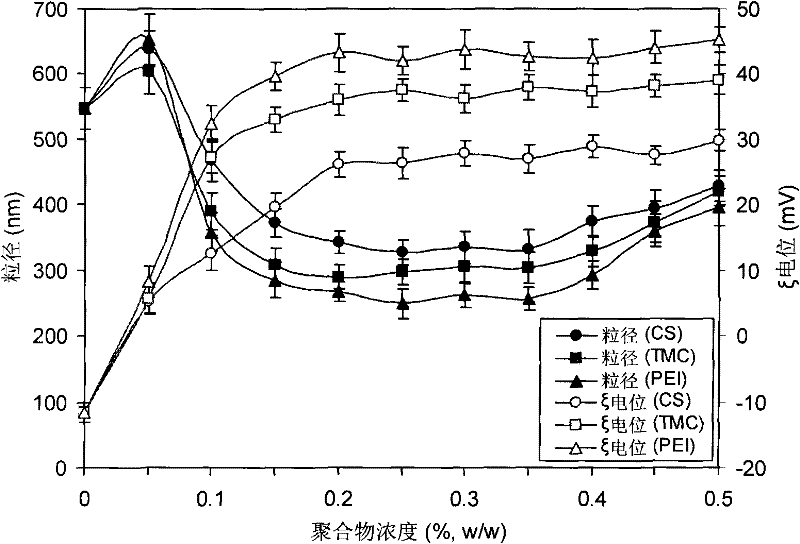
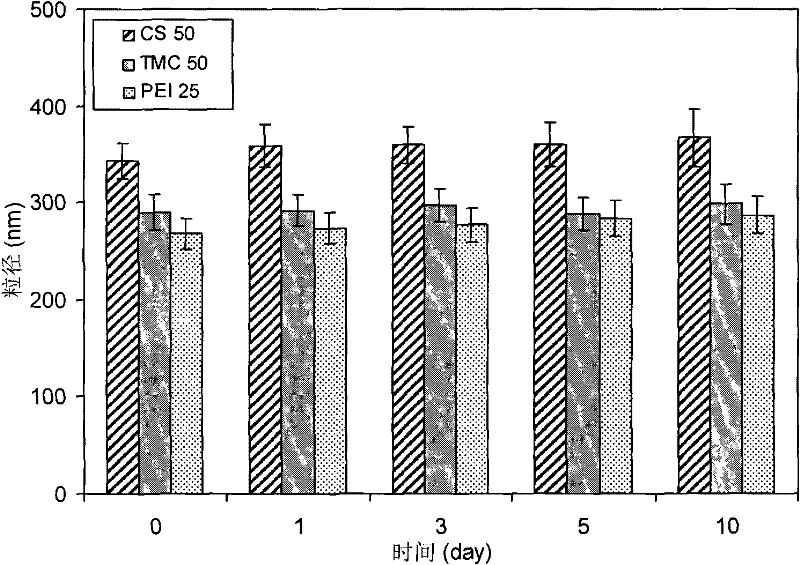
[0021] Place itraconazole 1% (w / v) in an aqueous solution containing poloxamer 1880.5% and chitosan 0.2%, and ultrasonically disperse to obtain a coarse dispersion system of itraconazole. The chitosan is chitosan with a molecular weight of 50kDa and a deacetylation degree of 86.5%. The obtained coarse dispersion system is pre-circulated twice at 100, 300, 500, 800, 1000 and 1200bar by using a high-pressure homogenizer, and circulated at 1400bar for 20 times to obtain itraconazole nanosuspension, which is diluted with water to an appropriate Concentration, its particle diameter measured by laser particle size analyzer is 342nm, and ξ potential is 26mV, and this system can be stable more than 10 days ( figure 2 ). Compared with the formulation without chitosan, the particle size decreased by 37%. ...
Embodiment 2
[0023] Itraconazole Nanosuspension
[0024] Itraconazole 1.0g
[0025] Poloxamer 188 0.5g
[0026] Trimethyl Chitosan 0.5g
[0027] Distilled water 100ml
[0028] Preparation Process:
[0029] Put itraconazole 1% (w / v) in an aqueous solution containing poloxamer 1880.5% and trimethyl chitosan 0.5%, and ultrasonically disperse to obtain a coarse dispersion system of itraconazole. The trimethyl chitosan is a trimethyl chitosan with a molecular weight of 50kDa and an amino substitution degree of 40%. The obtained coarse dispersion system is pre-circulated twice at 100, 300, 500, 800, 1000 and 1200bar by using a high-pressure homogenizer, and circulated at 1400bar for 20 times to obtain itraconazole nanosuspension, which is diluted with water to an appropriate Concentration, the laser particle size analyzer measures its particle size as 421nm, and the ξ potential is 39mV, and the system can be stable for more than 10 days. Compared with the formulation without trimethyl chit...
Embodiment 3
[0031] Itraconazole Nanosuspension
[0032] Itraconazole 1.0g
[0033] Poloxamer 188 0.5g
[0034] Polyethyleneimine 0.1g
[0035] Distilled water 100ml
[0036] Preparation Process:
[0037] Put itraconazole 1% (w / v) in an aqueous solution containing poloxamer 18880.5% and polyethyleneimine 0.1%, and ultrasonically disperse to prepare a coarse dispersion system of itraconazole. The polyethyleneimine is a branched polyethyleneimine with a molecular weight of 25kDa. The obtained coarse dispersion system is pre-circulated twice at 100, 300, 500, 800, 1000 and 1200bar by using a high-pressure homogenizer, and circulated at 1400bar for 20 times to obtain itraconazole nanosuspension, which is diluted with water to an appropriate Concentration, the laser particle size analyzer measures its particle size as 358nm, and the ξ potential is 32mV. The system can be stable for more than 10 days. Compared with the formulation without polyethyleneimine, the particle size decreased by 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com