Method for preparing a halogenocetyl fluoride and the derivatives thereof
A technology of haloacetyl fluoride and haloacetyl halide, which is applied in the field of preparation of haloacetyl fluoride and its derivatives, and can solve the problems of long reaction time and selectivity reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] 500 g of PER was charged into a 2 L jacketed Pyrex reactor with a condenser (-10 °C) and a mercury vapor lamp in a gas stream containing a mixture of pure oxygen (4 l / h) and nitrogen (0.5 l / h) under reflux (110°C) under radiation (210-260 nm).
[0147] A gas chromatographic analysis of the reaction medium (gas analysis detection) after 4 h of reaction showed the presence of 74% TCAC and 26% PER (% by weight).
[0148] Add 210.6 g (0.86 mol) of TCAC / PER (74 / 26 w / w) mixture and 40 g (2 mol) of anhydrous hydrofluoric acid into 0.3 liter of Hastelloy cooled to 0°C C276 autoclave.
[0149] The reactor was then heated to 120°C (temperature increase in about 1 h) and then kept at 120°C for 5 h under autogenous pressure (pressure about 60 bar).
[0150] The reactor was then cooled to 0°C (residual pressure about 15 bar), and the reaction medium was then slowly pumped into a polyvinyl fluoride flask cooled to -30°C.
[0151] The biphasic reaction medium is then degassed with...
Embodiment 2 to 4
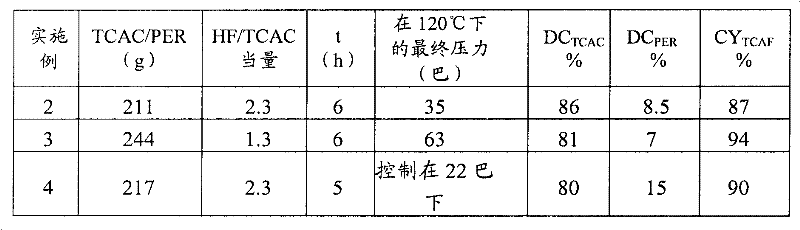
[0179] The following examples involving the fluorination of TCAC to give TCAF were carried out under the same general conditions (temperature = 120° C.); only the changed parameters are mentioned in the table (I) below.
[0180] In Examples 3 and 4, the reaction was carried out at autogenous pressure, in Example 4 the control pressure was 22 bar.
[0181] The results obtained are reported in the table below:
[0182] Table (I)
[0183]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com