Process for producing graphite fluoride by low-temperature intercalation method
A technology of fluorinated graphite and intercalated graphite, applied in the chemical industry, can solve the problems of difficulty in large-scale production, danger, and relatively little research on the production process of fluorinated graphite, and achieve the effect of avoiding harsh requirements and avoiding danger.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: The synthesis of graphite fluoride has three steps:
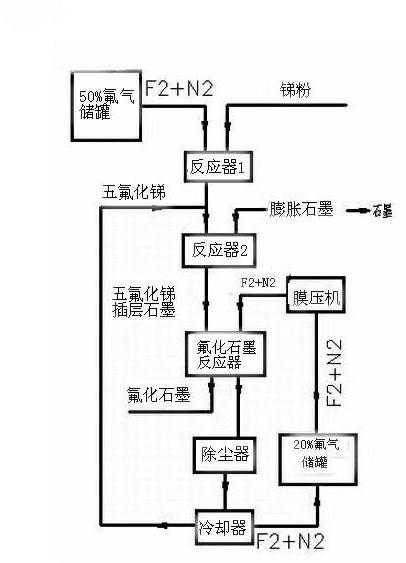
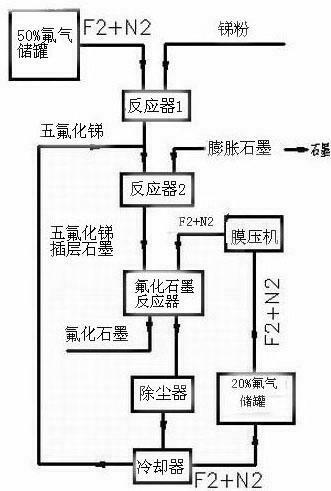
[0015] The production of graphite fluoride is divided into three steps: as figure 1 Shown:
[0016] In the first step, high-purity antimony pentafluoride is prepared by direct reaction of fluorine-nitrogen mixed gas with 50% mass percentage concentration of fluorine and nitrogen and antimony powder; the reaction equation is: 5F 2 +2Sb = 2SbF 5 .
[0017] Fluorine and nitrogen accounted for 50% of the mass percentage concentration of fluorine and nitrogen mixed gas, the flow rate is controlled by the fluorine gas flow meter, and enters the reactor from the reactor nozzle; The speed adjusts the feed amount; antimony powder and fluorine-nitrogen mixed gas directly react to generate gaseous antimony pentafluoride, and the gaseous antimony pentafluoride generated by the reaction is cooled by a cooler to obtain liquid high-purity antimony pentafluoride;
[0018] In the second step, liquid high-purity antim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com