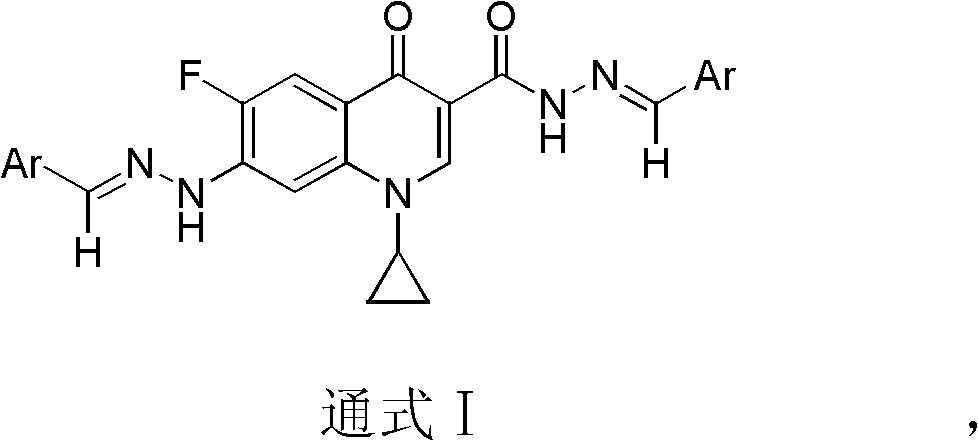
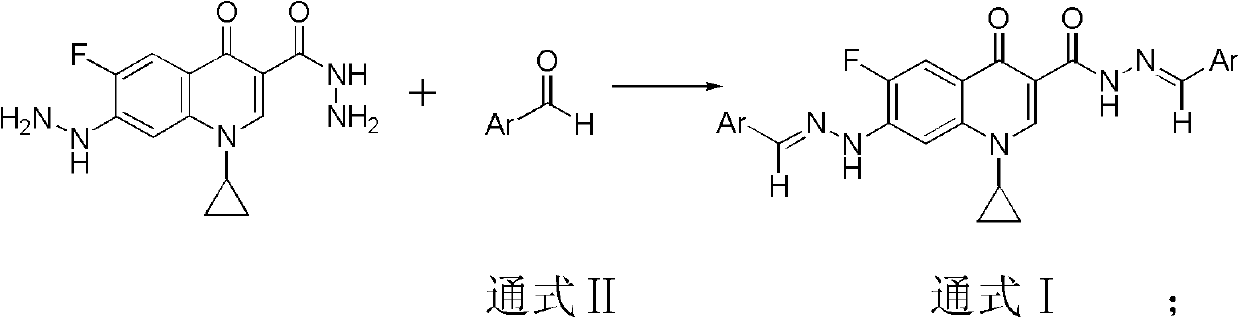
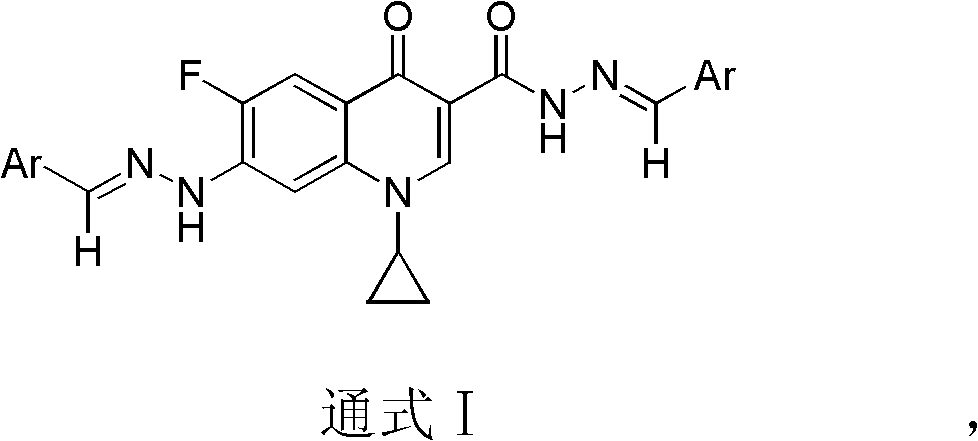
Ciprofluoroquinolone C3/C7 dithizone Schiff base, preparation method and application thereof
A technology of ciprofluoroquinolone and Schiff base, which is applied in the field of medicine, can solve the problems that ciprofluoroquinolone has not yet been seen, and achieve the effect of strong anti-tumor activity and strong in vitro cytotoxic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-15
[0018] Examples 1-15 are specific examples of ciprofluoroquinolone C3 / C7 dihydrazone Schiff base.
Embodiment 1
[0020] The Cyprofluoroquinolone C3 / C7 dihydrazone Schiff base provided in this example is benzaldehyde acetal (1-cyclopropyl-6-fluoro-7-hydrazino-quinolin-4(1H)-one-3-methanol Hydrazide) dihydrazone, its structural formula is:
[0021]
Embodiment 2
[0023] The Cyprofluoroquinolone C3 / C7 dihydrazone Schiff base provided in this example is p-tolualdehyde acetal (1-cyclopropyl-6-fluoro-7-hydrazino-quinolin-4(1H)-one- 3-formylhydrazide) dihydrazone, its structural formula is:
[0024]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com