Furyl alpha-aminophosphonate chitosan derivative and its preparation method
A technology of chitosan derivatives and amino phosphonates, which is applied in botany equipment and methods, chemicals for biological control, biocides, etc., can solve the problems of limited application range, achieve good solubility, The effect of expanding the application field and avoiding the use of organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 derivative 1
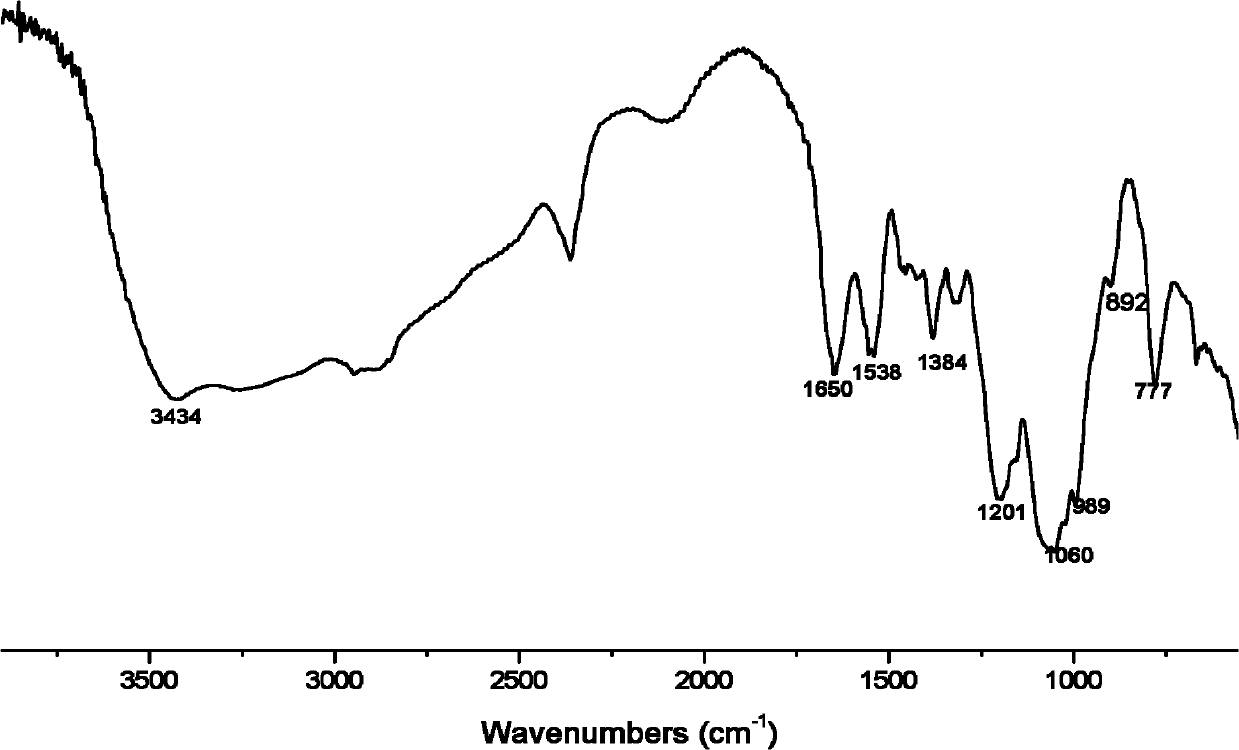
[0025] Add 10 mmol of chitosan with a molecular weight of 230,000, 10 mmol of dimethyl phosphite and 10 mmol of furfural into an agate mortar, grind and mix evenly, and react in a microwave reactor at 200W for 3 minutes. The product was soaked in ethanol for 1 hour, filtered with suction, washed with absolute ethanol, and dried at 60°C to obtain a brown powder, namely chitosan furanyl α-aminophosphonic acid methyl ester, ie derivative 1, see general formula I for the structural formula.
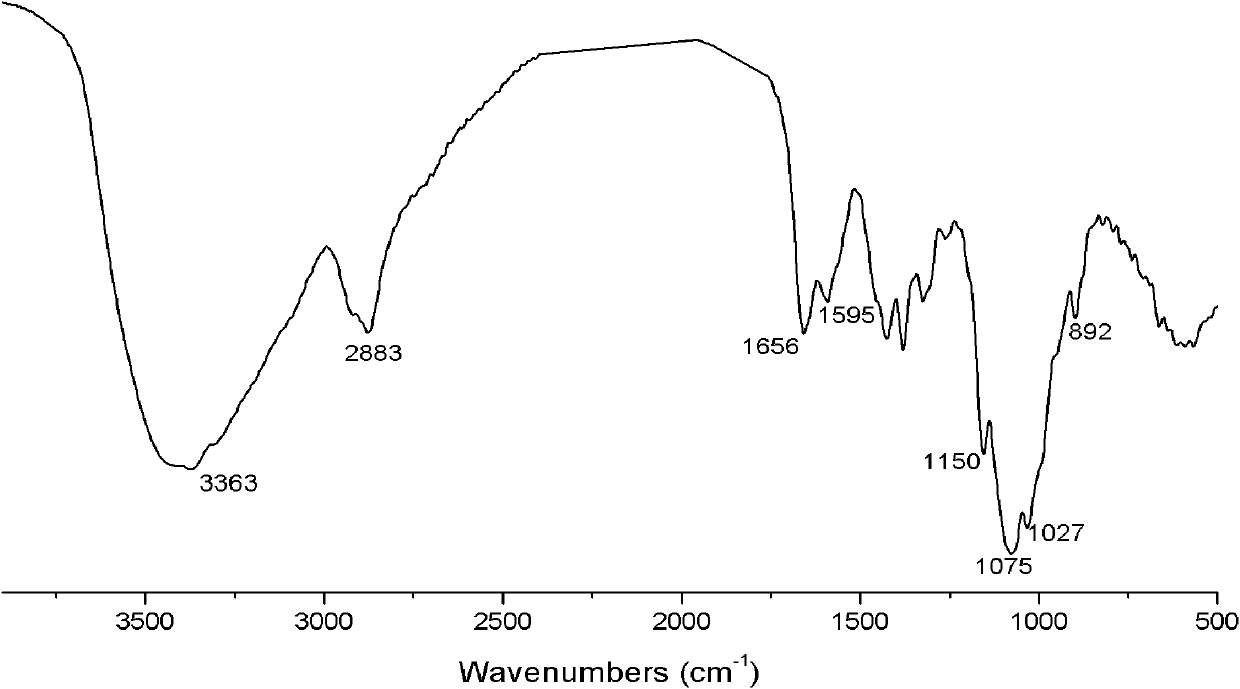
[0026] Infrared spectrum shows: the infrared spectrum of chitosan derivative 1 ( figure 2 ) and the infrared spectrum of chitosan ( figure 1 ) compared to that located at 1595cm -1 The characteristic absorption peak of NH disappears, indicating that NH 2 Has reacted; 1538, 1384cm -1 It is the characteristic absorption peak of N-H and C-N, 1201, 1060, 989cm -1 It is the characteristic absorption peak of P=O, P-O-C and P-C; it proves t...
Embodiment 2
[0027] The preparation of embodiment 2 derivative 2
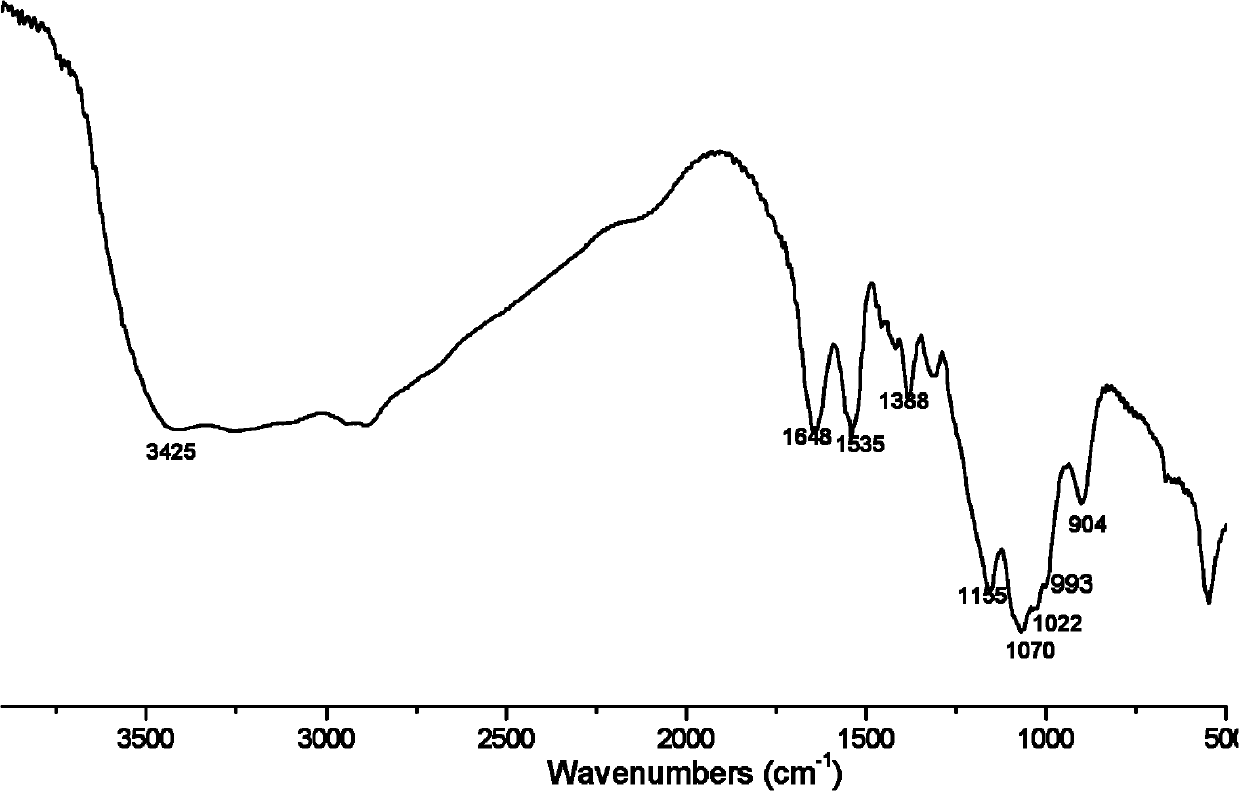
[0028] Add 10 mmol of chitosan with a molecular weight of 230,000, 10 mmol of diethyl phosphite, and 10 mmol of furfural into an agate mortar, grind and mix evenly, and react in a microwave reactor at 200W for 3 minutes. The product was soaked in ethanol for 1 hour, filtered with suction, washed with absolute ethanol, and dried at 60°C to obtain a brown powder, namely chitosan furanyl α-aminophosphonic acid ethyl ester, ie derivative 2, see general formula I for the structural formula.
[0029] Infrared spectrum shows: the infrared spectrum of chitosan derivative 2 ( image 3 ) and the infrared spectrum of chitosan ( figure 1 ) compared to that located at 1595cm -1 The characteristic absorption peak of NH disappears, indicating that NH 2 Has reacted; 1535, 1388cm -1 It is the characteristic absorption peak of N-H and C-N, 1155, 1070, 993cm -1 It is the characteristic absorption peaks of P=O, P-O-C and P-C; it proves th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com