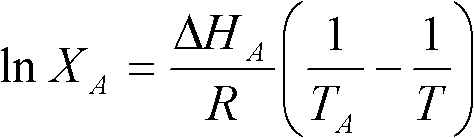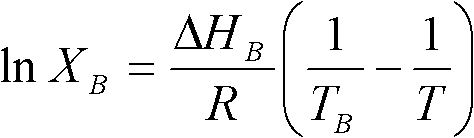Poly fatty acid shape-stabilized phase change material and preparation method thereof
A technology of shape-setting phase change materials and polybasic fatty acids, which is applied in the production of ceramic materials, materials for heat exchange, chemical instruments and methods, etc. , the effect of increasing the adsorption capacity and improving the energy saving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) According to Schroeder's formula, the optimal mixing mass ratio of myristic acid and stearic acid is calculated to be 72.66%: 27.34%, and myristic acid and stearic acid are mixed according to the proportion, and the two are mixed under the condition of 85 ℃ of water baths. The fatty acid mixture was heated and stirred for 50 minutes, and myristic acid / stearic acid binary eutectic mixture was obtained after ultrasonication for 3 minutes.
[0038] (2) Measure the binary eutectic mixture of myristic acid / stearic acid by differential scanning calorimetry, the phase transition temperature is 47.55°C, the phase transition latent heat is 182.3J / g, and its theoretical molecular mass is calculated to be 241.39; The dibasic fatty acid eutectic mixture is regarded as a single fatty acid, and then compounded with lauric acid. According to Schroeder's formula, the optimum mixing mass ratio of dibasic fatty acid eutectic mixture and lauric acid is 49% / 51%. ; After mixing accordin...
Embodiment 2
[0041] (1) Calculate the mixing mass ratio of lauric acid and myristic acid according to Schroeder's formula to be 65.08%: 34.92%, mix lauric acid and myristic acid according to the proportion, and mix the mixture of these two fatty acids under 65°C water bath condition Heated and stirred for 60 minutes, and ultrasonicated for 2 minutes to obtain a binary eutectic mixture of lauric acid / myristic acid.
[0042] (2) Measure the binary eutectic mixture of lauric acid / myristic acid by differential scanning calorimetry, the phase transition temperature is 35.10°C, the latent heat of phase transition is 210.2J / g, and its theoretical molecular mass is calculated to be 209.30; The dibasic fatty acid eutectic mixture is regarded as a single fatty acid, and then compounded with capric acid. According to Schroeder's formula, the optimal mixing mass ratio of dibasic fatty acid eutectic mixture and capric acid is 47.6% / 52.4% ; After mixing according to the ratio, heat and stir it in a wate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
| Latent heat of phase change | aaaaa | aaaaa |
| Phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com