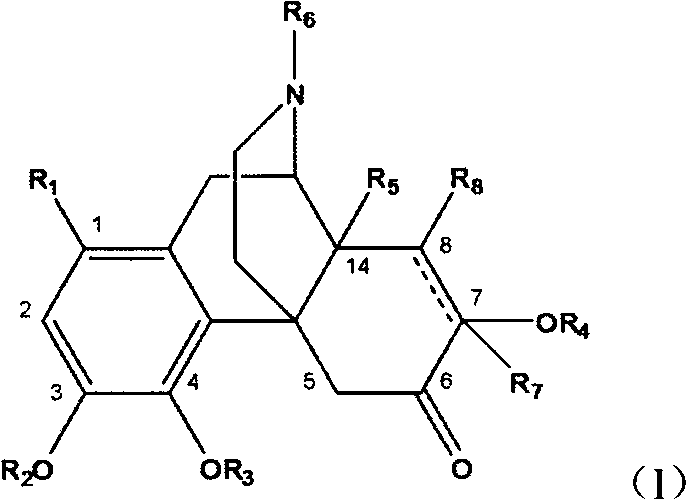
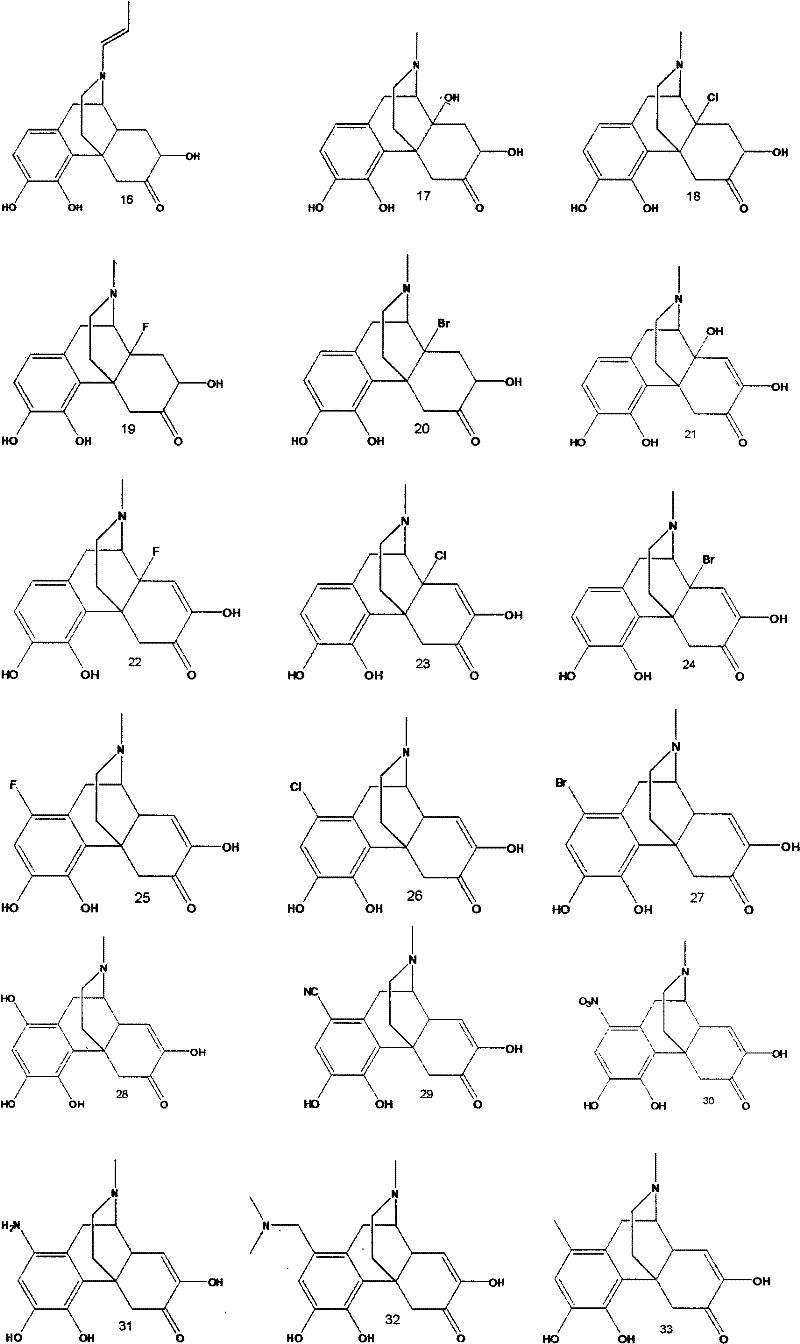
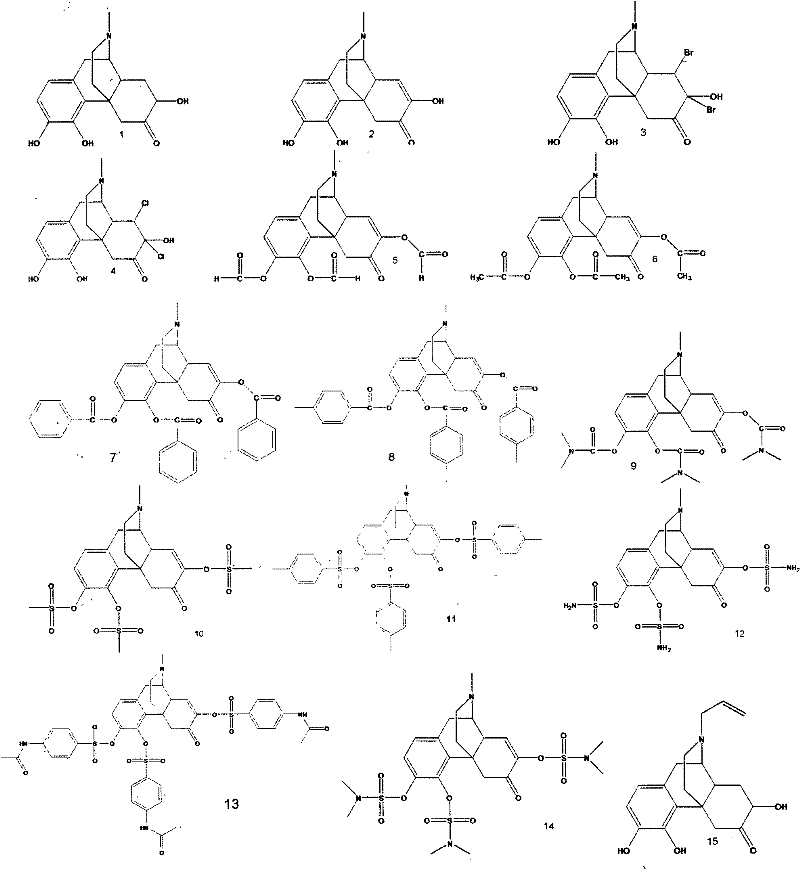Sinomenine derivative
A compound, selected technology, used in the application of anti-inflammatory or antitussive drugs in the treatment of diseases, analgesia, sinomenine derivatives and pharmaceutically acceptable salts thereof, can solve adverse reactions, dosage oversized issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Synthesis of Compound 3
[0106]
[0107] Dissolve 3.3g of sinomenine in 100mL of ethyl acetate, then add 1.6g of bromine to the solution, stir at room temperature for 30min, add 20mL of water, transfer to a separatory funnel, shake and mix well, let stand to separate layers, and separate acetic acid Add 2.0 g of boron bromide to the ethyl acetate layer, stir at room temperature for 1 h, filter with suction, recover ethyl acetate under reduced pressure to 25 mL, and crystallize to obtain compound 3 with a yield of 89.52%.
Embodiment 2
[0109] Synthesis of Compound 4
[0110]
[0111] Dissolve 3.3g of sinomenine in 120mL of ethyl acetate, then inject chlorine gas into the solution, stir at room temperature for 30min, add 20mL of water, transfer to a separatory funnel, shake and mix well, let stand to separate layers, and separate ethyl acetate Add 3.2 g of boron bromide to the ethyl acetate layer, stir at room temperature for 1 h, filter with suction, recover ethyl acetate under reduced pressure to 25 mL, and crystallize to obtain compound 4 with a yield of 91.7%.
Embodiment 3
[0113] Synthesis of Compound 2
[0114]
[0115] Dissolve 4.6 g of compound 3 in 30 mL of absolute ethanol, then add zinc powder to the solution, stir at room temperature for 120 min, filter with suction, recover ethanol to 15 mL under reduced pressure, refrigerate at 4°C for 12 h, and crystallize to obtain compound 2 with a yield of 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com